Catalog No.
DHD46601
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
CD73, 5'-nucleotidase, NT5E, NT5, 5'-NT, Ecto-5'-nucleotidase, NTE
Concentration
1.31 mg/ml
Endotoxin level
Please contact with the lab for this information.
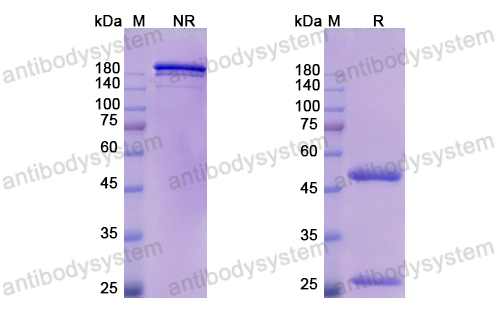
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P21589
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MEDI-9447, CAS: 1803176-05-7
Clone ID
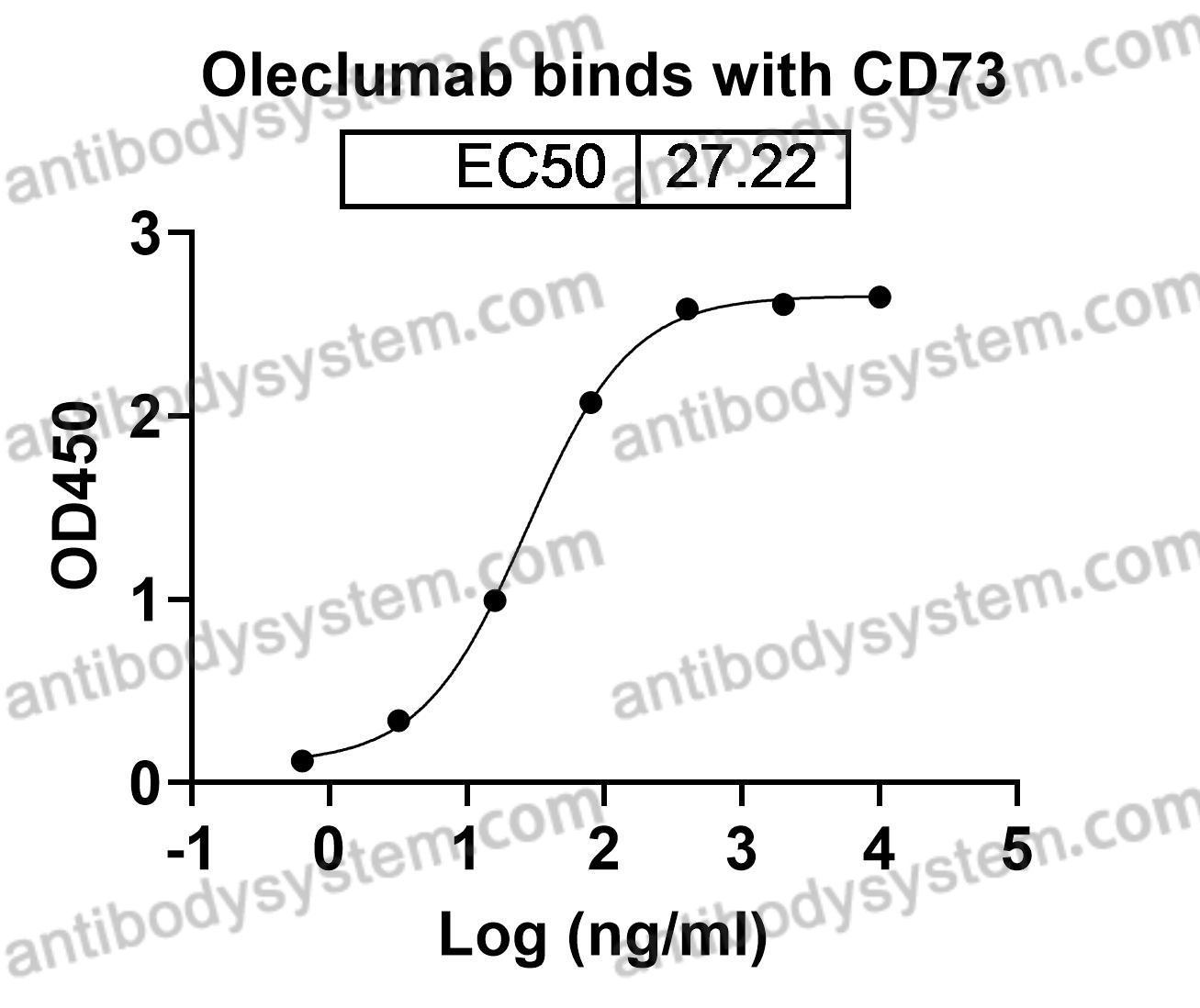
Oleclumab
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Adapting radiation therapy to immunotherapy: Delineation and treatment planning of pre-operative immune-modulating breast iSBRT in 151 patients treated in the randomized phase II Neo-CheckRay trial., PMID:40057199
Continuous replenishment of the dysfunctional CD8 T cell axis is associated with response to chemoimmunotherapy in advanced breast cancer., PMID:39983715
Peripheral blood leukocyte signatures as biomarkers in relapsed ovarian cancer patients receiving combined anti-CD73/anti-PD-L1 immunotherapy in arm A of the NSGO-OV-UMB1/ENGOT-OV30 trial., PMID:39887612
A Phase Ib/II Randomized Clinical Trial of Oleclumab with or without Durvalumab plus Chemotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma., PMID:39106081
COLUMBIA-1: a randomised study of durvalumab plus oleclumab in combination with chemotherapy and bevacizumab in metastatic microsatellite-stable colorectal cancer., PMID:39048638
PACIFIC-9: Phase III trial of durvalumab + oleclumab or monalizumab in unresectable stage III non-small-cell lung cancer., PMID:39023287
NSGO-OV-UMB1/ENGOT-OV30: A phase II study of durvalumab in combination with the anti-CD73 monoclonal antibody Oleclumab in patients with relapsed ovarian cancer., PMID:38943691
A phase 2 study of AZD4635 in combination with durvalumab or oleclumab in patients with metastatic castration-resistant prostate cancer., PMID:38430405
Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial., PMID:38351187
Efficacy and pharmacodynamic effect of anti-CD73 and anti-PD-L1 monoclonal antibodies in combination with cytotoxic therapy: observations from mouse tumor models., PMID:38206570
Author Correction: Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:38086864
First-in-human study of SBRT and adenosine pathway blockade to potentiate the benefit of immunochemotherapy in early-stage luminal B breast cancer: results of the safety run-in phase of the Neo-CheckRay trial., PMID:38056900
Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:37919269
Bispecific antibody CD73xEGFR more selectively inhibits the CD73/adenosine immune checkpoint on cancer cells and concurrently counteracts pro-oncogenic activities of CD73 and EGFR., PMID:37734877
Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial., PMID:37707791
A Novel Bispecific Antibody for EpCAM-Directed Inhibition of the CD73/Adenosine Immune Checkpoint in Ovarian Cancer., PMID:37509310
Tumor intrinsic and extrinsic functions of CD73 and the adenosine pathway in lung cancer., PMID:37033953
First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors., PMID:37016126
CD73 Inhibitor Oleclumab Plus Osimertinib in Previously Treated Patients With Advanced T790M-Negative EGFR-Mutated NSCLC: A Brief Report., PMID:36641093
Pharmacology, pharmacokinetics, and toxicity characterization of a novel anti-CD73 therapeutic antibody IBI325 for cancer immunotherapy., PMID:36587633
Safety, tolerability, pharmacokinetics, and antitumour activity of oleclumab in Japanese patients with advanced solid malignancies: a phase I, open-label study., PMID:36342599
Current challenges of unresectable stage III NSCLC: are we ready to break the glass ceiling of the PACIFIC trial?, PMID:35923929
Adenosine pathway inhibitors: novel investigational agents for the treatment of metastatic breast cancer., PMID:35575038
COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer., PMID:35452273
Bispecific antibody CD73xEpCAM selectively inhibits the adenosine-mediated immunosuppressive activity of carcinoma-derived extracellular vesicles., PMID:34464670
Neo-CheckRay: radiation therapy and adenosine pathway blockade to increase benefit of immuno-chemotherapy in early stage luminal B breast cancer, a randomized phase II trial., PMID:34362344