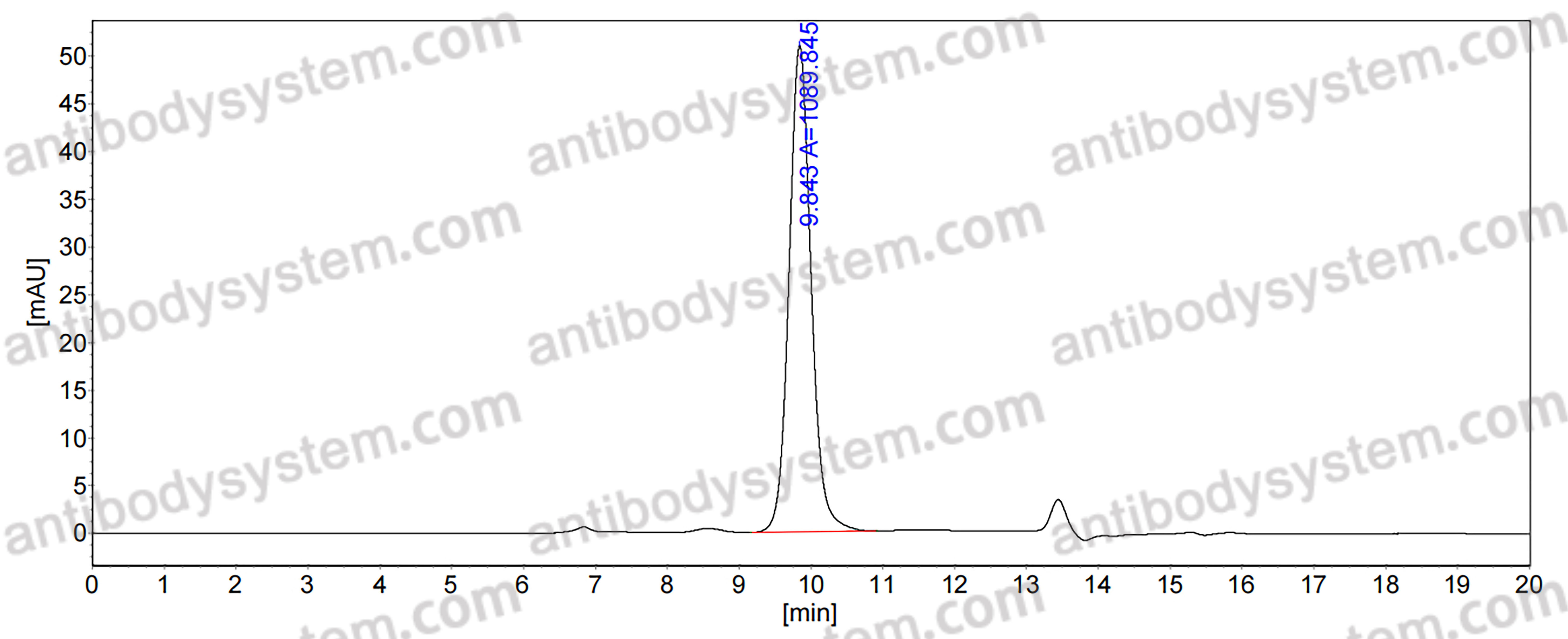
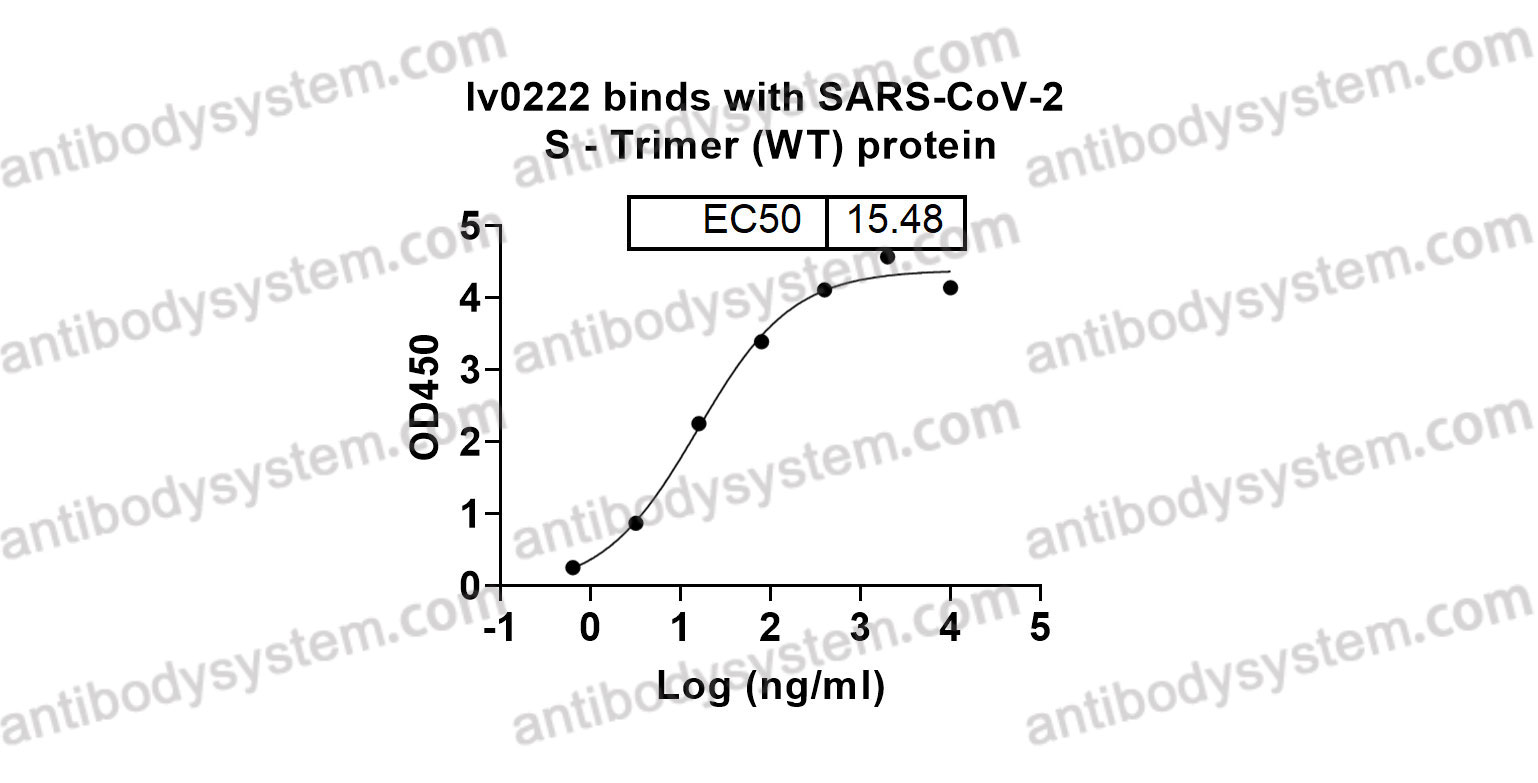
Design of SARS-CoV-2 RBD immunogens to focus immune responses toward conserved coronavirus epitopes., PMID:40511920
Conformational Landscaping and Dynamic Mutational Profiling of Binding Interactions and Immune Escape for Broadly Neutralizing Class I Antibodies with SARS-CoV-2 Spike Protein: Distributed Binding Hotspot Networks Underlie Mechanism of Viral Resistance Against Existing Variants., PMID:40510568
Nonstabilized SARS-CoV-2 spike mRNA vaccination induces broadly neutralizing antibodies in nonhuman primates., PMID:40498855
Immune response induced by the recombinant novel coronavirus vaccine (Adenovirus type 5 vector) (Ad5-nCoV) in persons living with HIV (PLWH)., PMID:40498774
Viral evolution prediction identifies broadly neutralizing antibodies to existing and prospective SARS-CoV-2 variants., PMID:40494884
Structural Analysis of the SARS-CoV-2 Spike N-Terminal Domain Across Wild-Type and Recent Variants: A Comparative Study., PMID:40485545
GWAS identifies genetic loci for antibody response to SARS-CoV-2 vaccines in patients with systemic autoimmune diseases and healthy individuals., PMID:40463545
Phylogeny-driven design of broadly protective sarbecovirus receptor-binding domain nanoparticle vaccines., PMID:40463102
Allostery Links hACE2 Binding, Pan-variant Neutralization and Helical Extension in the SARS-CoV-2 Spike Protein., PMID:40447075
Multiplex ACE2-RBD binding inhibition assay: An integrated tool for assessing neutralizing antibodies to SARS-CoV-2 variants and protection against breakthrough infections., PMID:40447072
Development of bivalent RBD adapted COVID-19 vaccines for broad sarbecovirus immunity., PMID:40442110
XBB.1.5 RBD-Based Bivalent Vaccines Induced Antibody Responses Against SARS-CoV-2 Variants in Mice., PMID:40432153
Comparison of Three Commercial ELISA Kits for Detection of Antibodies Against SARS-CoV-2 in Serum Samples from Different Animal Species., PMID:40431727
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
Is SARS-CoV-2 Spike Evolution Being Retargeted at the N-Terminal Domain?, PMID:40415355
Maternal Vaccination and Protective Immunity Against SARS-CoV-2 Infection in Pups., PMID:40411252
Evaluation of broad-spectrum protection by novel mRNA vaccines against SARS-CoV-2 variants (Delta, Omicron-BA.5, XBB-EG.5) in the golden hamster model., PMID:40410742
Comparative study of humoral and cellular immunity against SARS-CoV-2 induced by different COVID-19 vaccine types: Insights into protection against wildtype, Delta and JN.1 omicron strains., PMID:40408899
Cross-reactive sarbecovirus antibodies induced by mosaic RBD nanoparticles., PMID:40402246
Immunogenicity Evaluation of a SARS-CoV-2 BA.2 Subunit Vaccine Formulated with CpG 1826 plus alum Dual Adjuvant., PMID:40383947
Nanoparticle-supported, rapid, digital quantification of neutralizing antibodies against SARS-CoV-2 variants., PMID:40383030
Safety and immunogenicity of SARS-CoV-2 protein subunit recombinant vaccine (Indovac®) in healthy populations aged 18 years and above in Indonesia: A phase I, observer-blind, randomized, controlled study., PMID:40381203
A recombinant BCG with surface-displayed antigen induces humoral and cellular immune responses., PMID:40379714
Biophysics of SARS-CoV-2 spike protein's receptor-binding domain interaction with ACE2 and neutralizing antibodies: from computation to functional insights., PMID:40376405
Exploring Diverse Binding Mechanisms of Broadly Neutralizing Antibodies S309, S304, CYFN-1006 and VIR-7229 Targeting SARS-CoV-2 Spike Omicron Variants: Integrative Computational Modeling Reveals Balance of Evolutionary and Dynamic Adaptability in Shaping Molecular Determinants of Immune Escape., PMID:40376091
Comparative performance of serum and plasma samples in SARS-CoV-2 serology and neutralization assays., PMID:40374015
mRNA-delivered neutralizing antibodies confer protection against SARS-CoV-2 variant in the lower and upper respiratory tract., PMID:40342969
Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with lung infection and tissue-damage biomarkers., PMID:40339608
Low Antibody-Dependent Enhancement of Viral Entry Activity Supports the Safety of Inactivated SARS-CoV-2 Vaccines., PMID:40333308
Cryo-EM reveals conformational variability in the SARS-CoV-2 spike protein RBD induced by two broadly neutralizing monoclonal antibodies., PMID:40330036
Repeated-dose toxicity and immunogenicity evaluation of a recombinant subunit COVID-19 vaccine (ZF2001) in rats., PMID:40330020
Integrating immune library probing with structure-based computational design to develop potent neutralizing nanobodies against emerging SARS-CoV-2 variants., PMID:40329514
Temporal correlations between RBD-ACE2 blocking and binding antibodies to SARS-CoV-2 variants in CoronaVac-vaccinated individuals and their persistence in COVID-19 patients., PMID:40328892
Enhanced variant neutralization through glycan masking of SARS-CoV-2 XBB1.5 RBD., PMID:40326334
AI designed, mutation resistant broad neutralizing antibodies against multiple SARS-CoV-2 strains., PMID:40319133
A SARS-CoV and SARS-CoV-2 RBD Heterodimer Vaccine Candidate., PMID:40317517
Identification of a nanobody able to catalyze the destruction of the spike-trimer of SARS-CoV-2., PMID:40317451
Minimal Impact of Prior Common Cold Coronavirus Exposure on Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination or Infection Risk in Older Adults in Congregate Care., PMID:40313478
Structural insights into the receptor-binding domain of bat coronavirus ZXC21., PMID:40306273
A distinctive IGHV3-66 SARS-CoV-2 neutralizing antibody elicited by primary infection with an Omicron variant., PMID:40306272
Safety and immunogenicity of PHH-1V booster against SARS-CoV-2 variants, including omicron subvariants: Results from a phase IIb open-label extension study., PMID:40304691
SARS-CoV-2 Seroprevalence in Indoor House Cats From the Lisbon Area During the COVID-19 Pandemic, 2019-2021., PMID:40303070
Differential immunogenicity in people living with HIV with varying CD4 levels after bivalent mRNA COVID-19 booster vaccination., PMID:40299994
Comparative analysis of replication and immune evasion among SARS-CoV-2 subvariants BA.2.86, JN.1, KP.2, and KP.3., PMID:40298448
Vaccination with ancestral SARS-CoV-2 spike adjuvanted with TLR agonists provides cross-protection against XBB.1., PMID:40295700
Automated and virus variant-programmable surrogate test qualitatively compares to the gold standard SARS-CoV-2 neutralization assay., PMID:40295688
Self-assembled epitope-based nanoparticles targeting the SARS-CoV-2 spike protein enhanced the immune response and induced potential broad neutralizing activity., PMID:40270771
Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV., PMID:40266218
Simian Immunodeficiency Virus-Based Virus-like Particles Are an Efficient Tool to Induce Persistent Anti-SARS-CoV-2 Spike Neutralizing Antibodies and Specific T Cells in Mice., PMID:40266067

-antibodysystem.jpg)