Catalog No.
DHE01901
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
CD27 ligand, CD70, TNFSF7, CD27LG, CD27L, CD70 antigen, Tumor necrosis factor ligand superfamily member 7, CD27-L
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
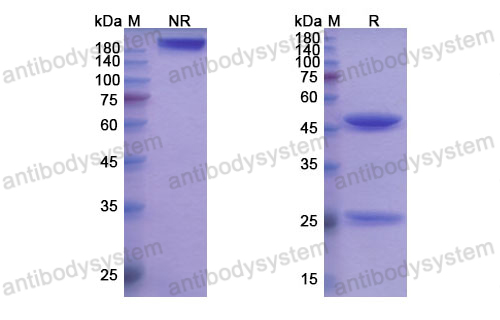
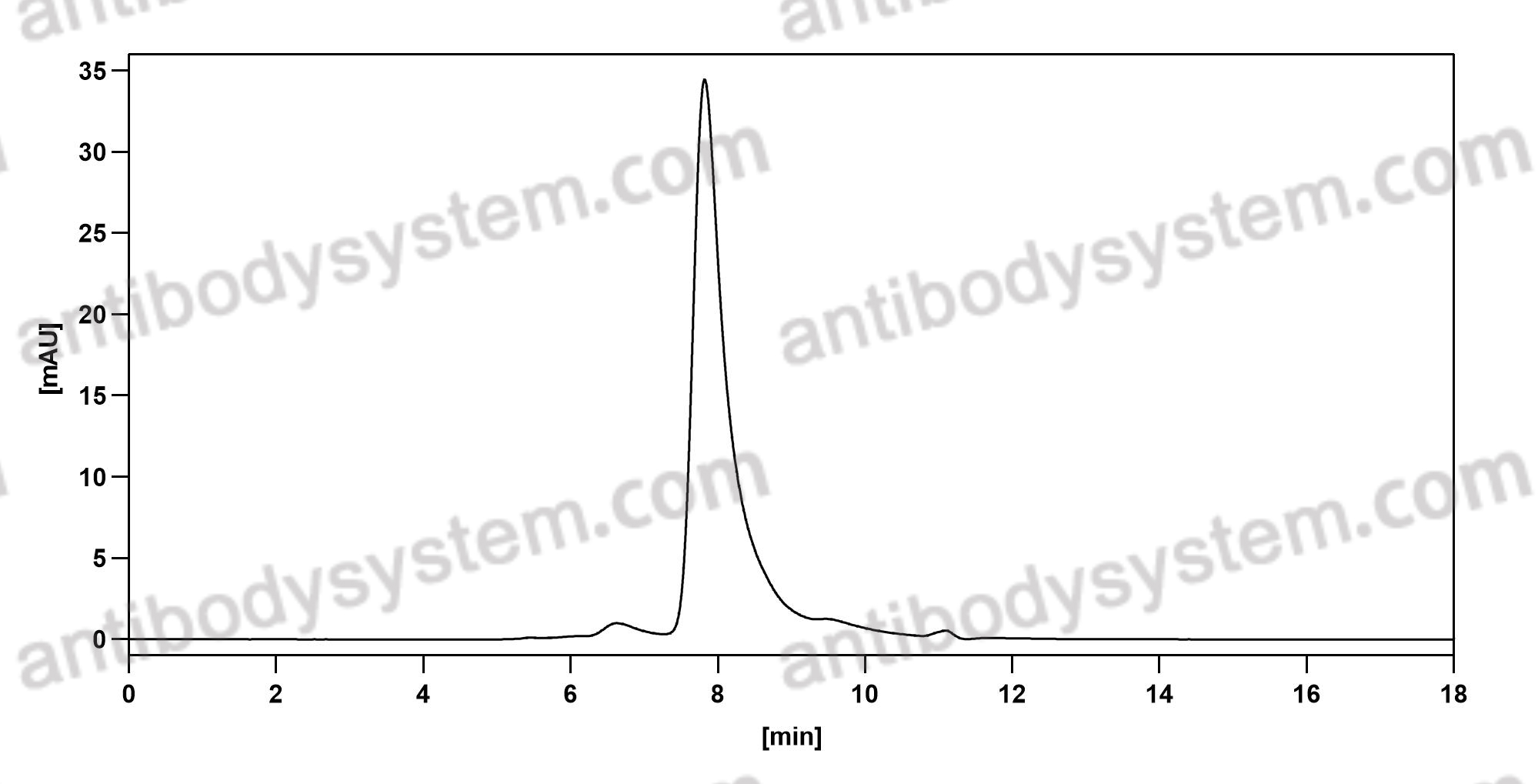
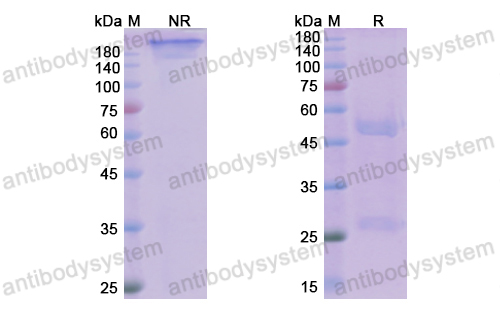
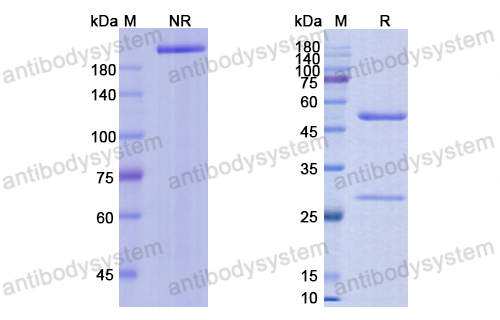
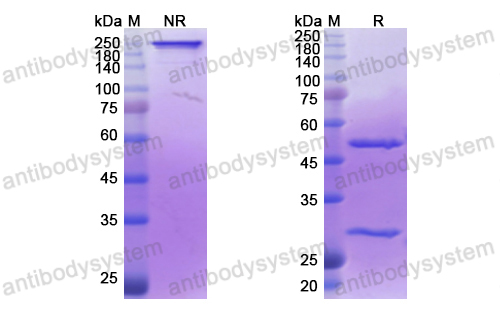
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P32970
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ARGX-110, CAS: 1399546-01-0
Clone ID
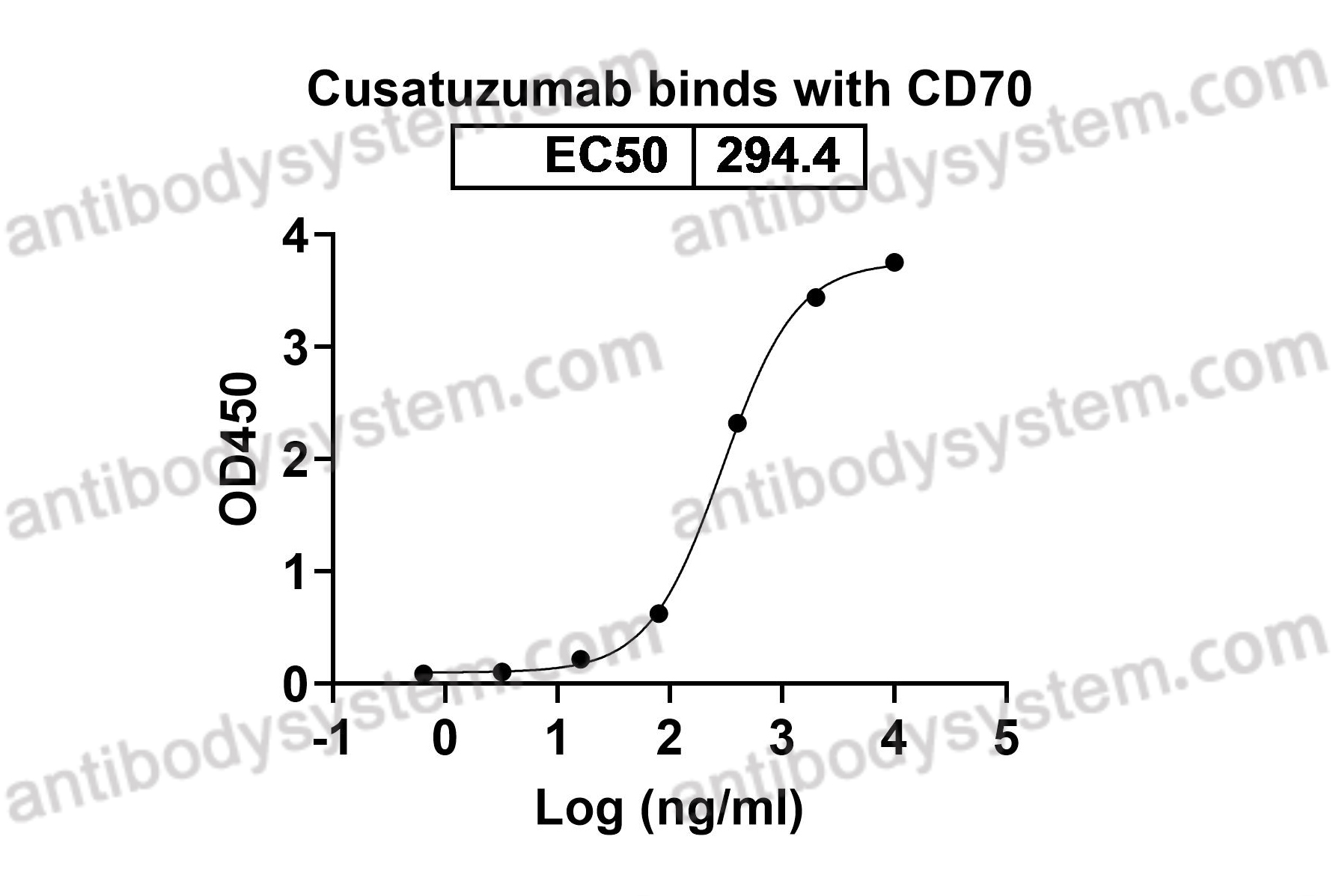
Cusatuzumab
Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents, PMID: 32601337
The CD70 Antibody Cusatuzumab Shows Promise in Acute Myeloid Leukemia, PMID: 34383699
An open-label, non-randomized, Phase Ib feasibility study of cusatuzumab in patients with nasopharyngeal carcinoma, PMID: 34405542
An Update on the Clinical Evaluation of Antibody-Based Therapeutics in Acute Myeloid Leukemia, PMID: 33630233
SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial, PMID: 34251873
Histopathological Markers for Target Therapies in Primary Cutaneous Lymphomas., PMID:37998391
Cusatuzumab plus azacitidine in newly diagnosed acute myeloid leukaemia ineligible for intensive chemotherapy (CULMINATE): part one of a randomised, phase 2, dose optimisation study., PMID:37914483
The novel high-affinity humanized antibody IMM40H targets CD70, eliminates tumors via Fc-mediated effector functions, and interrupts CD70/CD27 signaling., PMID:37849799
METTL3 inhibition induced by M2 macrophage-derived extracellular vesicles drives anti-PD-1 therapy resistance via M6A-CD70-mediated immune suppression in thyroid cancer., PMID:37648786
[Management of AML in the elderly]., PMID:36870810
Results from a phase I/II trial of cusatuzumab combined with azacitidine in patients with newly diagnosed acute myeloid leukemia who are ineligible for intensive chemotherapy., PMID:36779592
Cusatuzumab plus azacitidine in Japanese patients with newly diagnosed acute myeloid leukemia ineligible for intensive treatment., PMID:36394119
Cusatuzumab for treatment of CD70-positive relapsed or refractory cutaneous T-cell lymphoma., PMID:34726773
An open-label, nonrandomized, phase Ib feasibility study of cusatuzumab in patients with nasopharyngeal carcinoma., PMID:34405542
SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial., PMID:34251873
An Update on the Clinical Evaluation of Antibody-Based Therapeutics in Acute Myeloid Leukemia., PMID:33630233
Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents., PMID:32601337