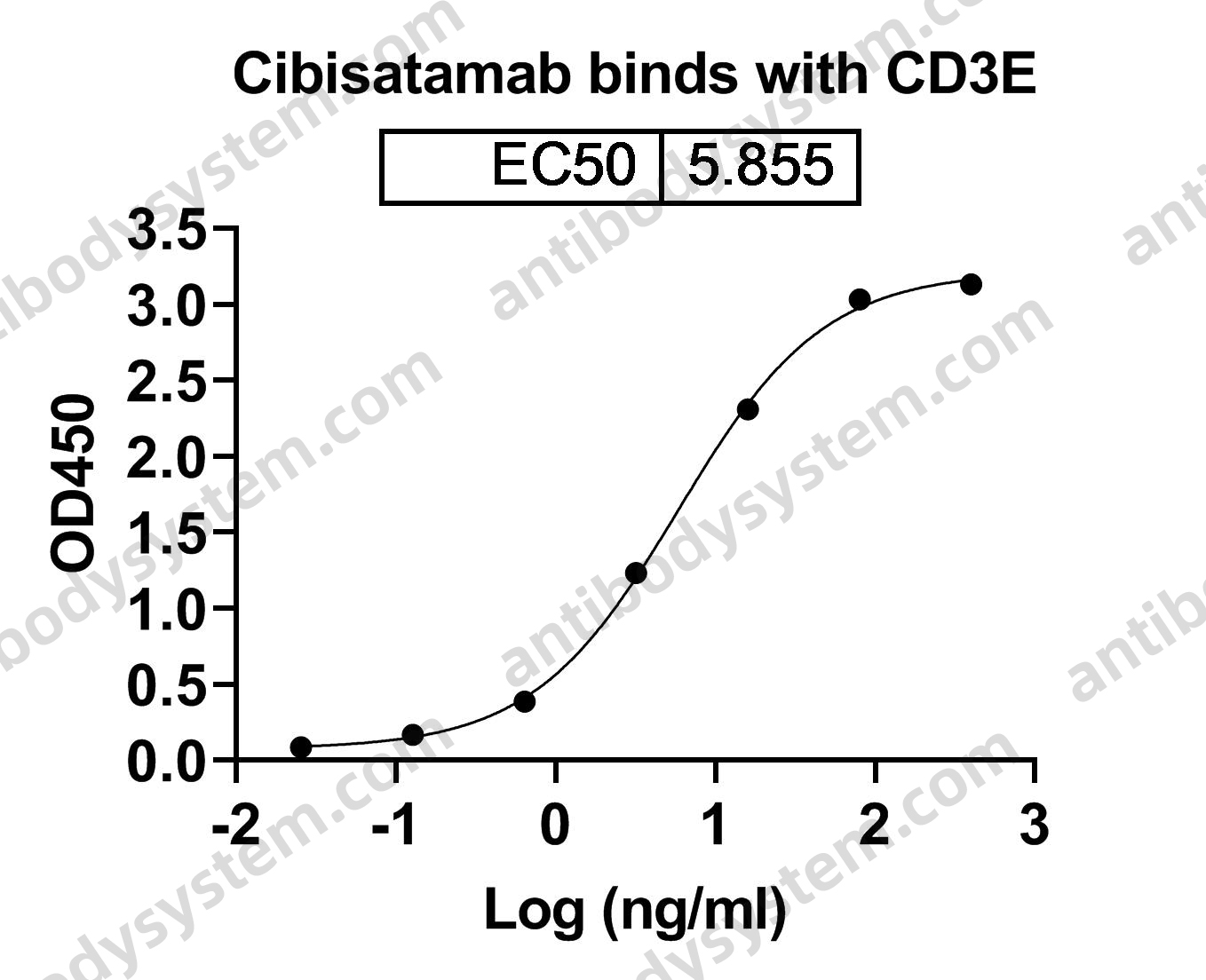
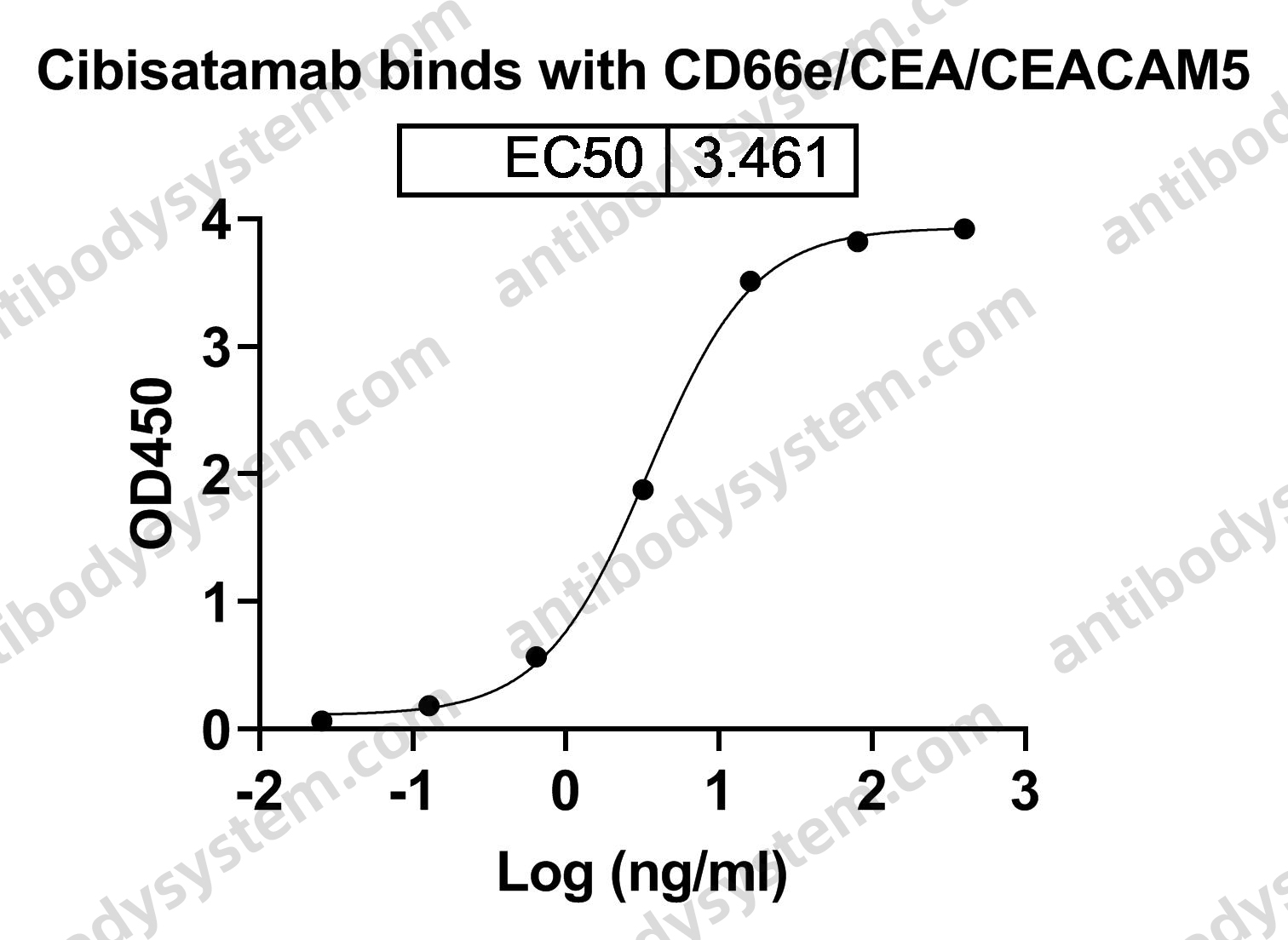
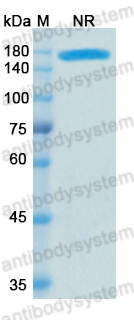
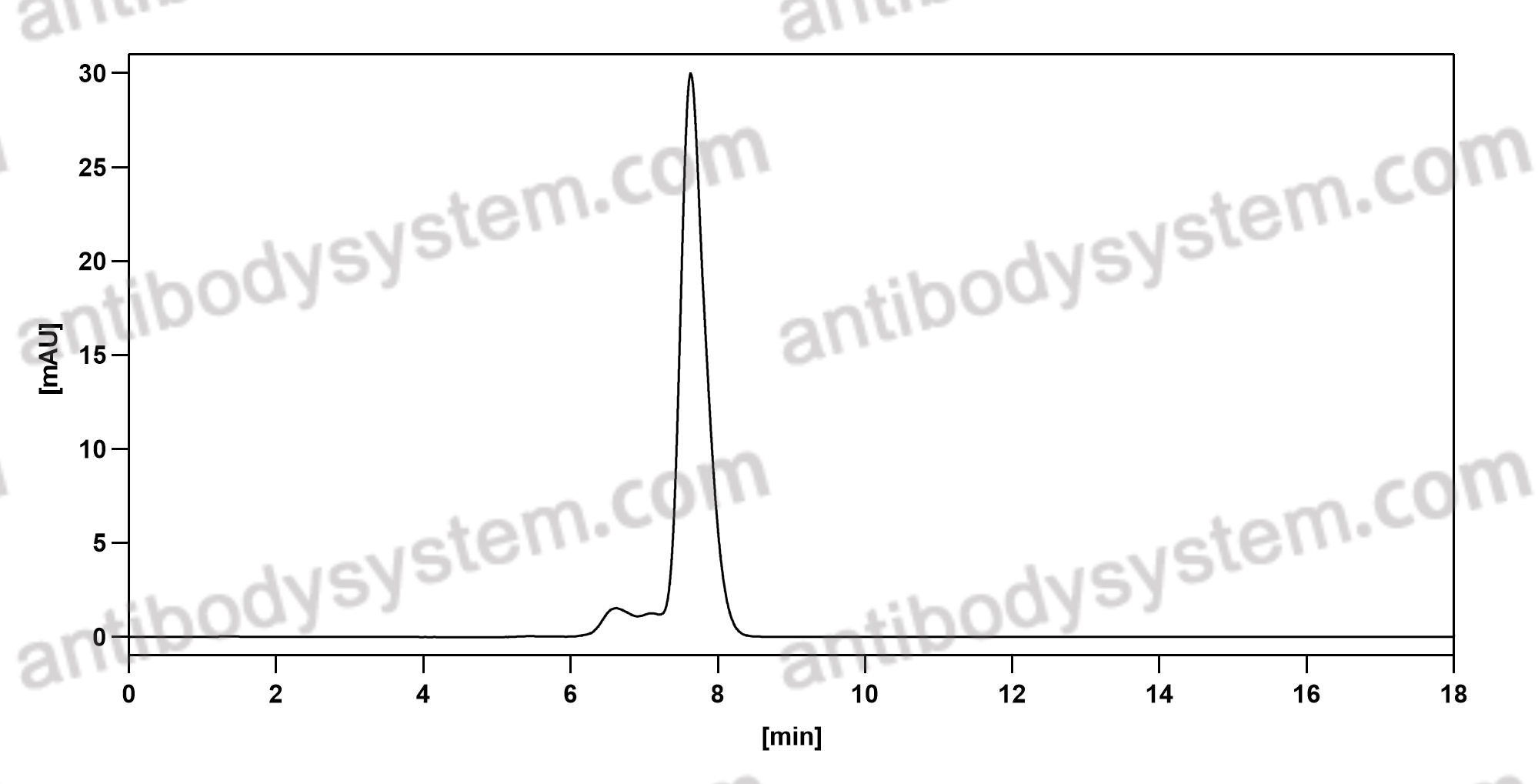
Catalog No.
DHC21003
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa/lambdawithdomaincrossover
Clonality
Monoclonal
Target
Meconium antigen 100, CEACAM5, CEA, CD66e, Carcinoembryonic antigen-related cell adhesion molecule 5, Carcinoembryonic antigen, T3E, T-cell surface antigen T3/Leu-4 epsilon chain, CD3e, CD3E, T-cell surface glycoprotein CD3 epsilon chain
Concentration
2.54 mg/ml
Endotoxin level
Please contact with the lab for this information.
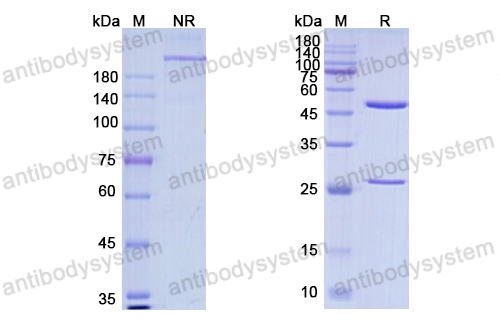
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P06731 & P07766
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Bispecific, CEA TCB, RG-7802, RO-6958688, CAS: 2101242-53-7
Clone ID
Cibisatamab
CEA expression heterogeneity and plasticity confer resistance to the CEA-targeting bispecific immunotherapy antibody cibisatamab (CEA-TCB) in patient-derived colorectal cancer organoids, PMID: 30982469
Predicting Tumor Killing and T-Cell Activation by T-Cell Bispecific Antibodies as a Function of Target Expression: Combining In Vitro Experiments with Systems Modeling, PMID: 33298591
A Model-Based Approach to Evaluate Anti-Drug Antibody Impact on Drug Exposure With Biologics: A Case Example With the CD3 T-Cell Bispecific Cibisatamab., PMID:40108739
In Vitro Evaluation of the Safety and Efficacy of Cibisatamab Using Adult Stem Cell-Derived Organoids and Colorectal Cancer Spheroids., PMID:39858076
Characterization of anti-drug antibody responses to the T-cell engaging bispecific antibody cibisatamab to understand the impact on exposure., PMID:38881900
CEA-CD3 bispecific antibody cibisatamab with or without atezolizumab in patients with CEA-positive solid tumours: results of two multi-institutional Phase 1 trials., PMID:38750034
A model-based approach leveraging in vitro data to support dose selection from the outset: A framework for bispecific antibodies in immuno-oncology., PMID:37964753
Using quantitative systems pharmacology modeling to optimize combination therapy of anti-PD-L1 checkpoint inhibitor and T cell engager., PMID:37408756
Three-dimensional colon cancer organoids model the response to CEA-CD3 T-cell engagers., PMID:35154495
Pharmacokinetics and Pharmacodynamics of T-Cell Bispecifics in the Tumour Interstitial Fluid., PMID:34959386
Multi-Parameter Quantitative Imaging of Tumor Microenvironments Reveals Perivascular Immune Niches Associated With Anti-Tumor Immunity., PMID:34421928
Predicting Tumor Killing and T-Cell Activation by T-Cell Bispecific Antibodies as a Function of Target Expression: Combining In Vitro Experiments with Systems Modeling., PMID:33298591
CEA expression heterogeneity and plasticity confer resistance to the CEA-targeting bispecific immunotherapy antibody cibisatamab (CEA-TCB) in patient-derived colorectal cancer organoids., PMID:30982469