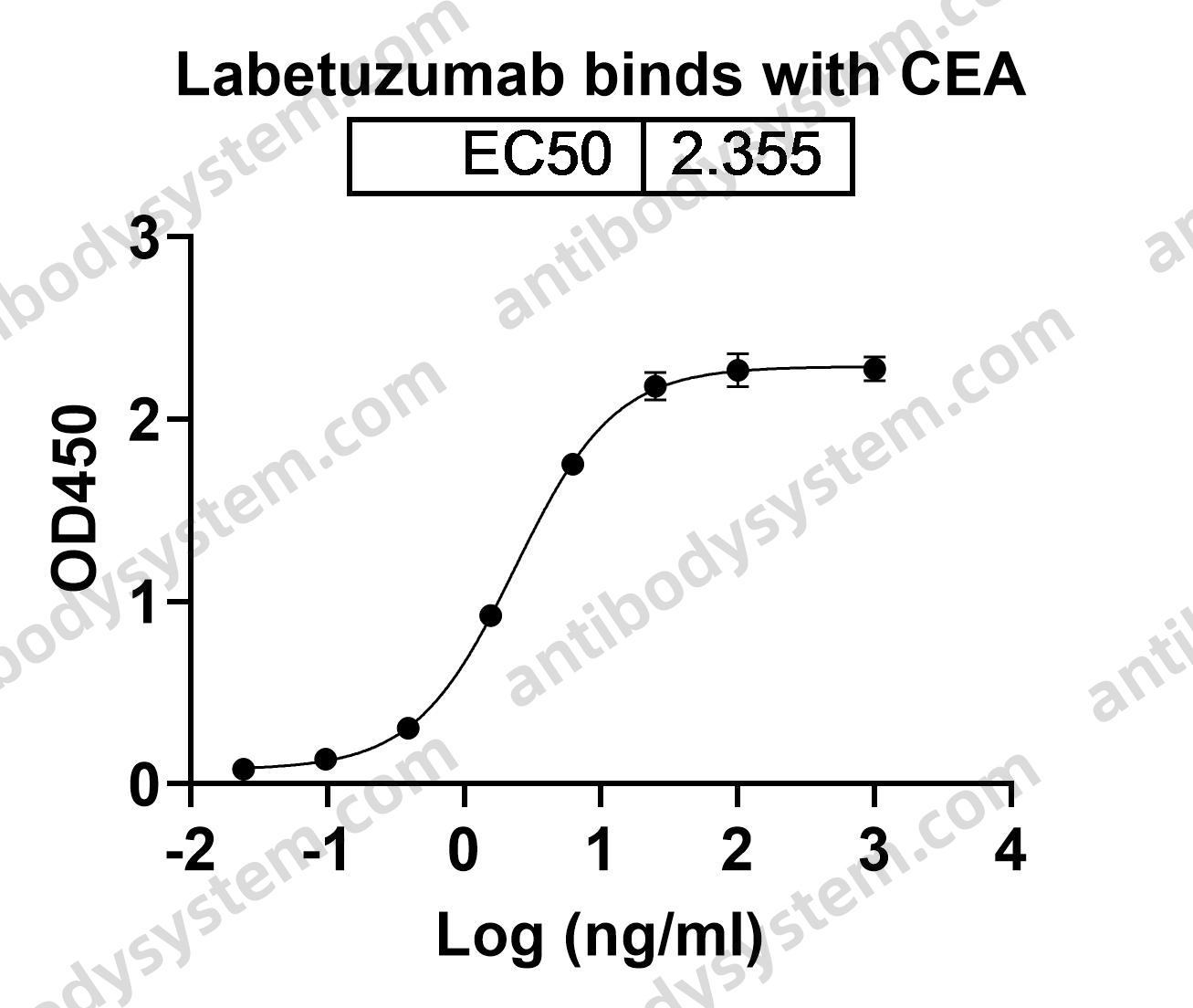
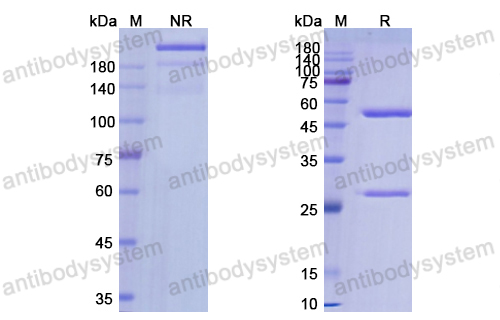
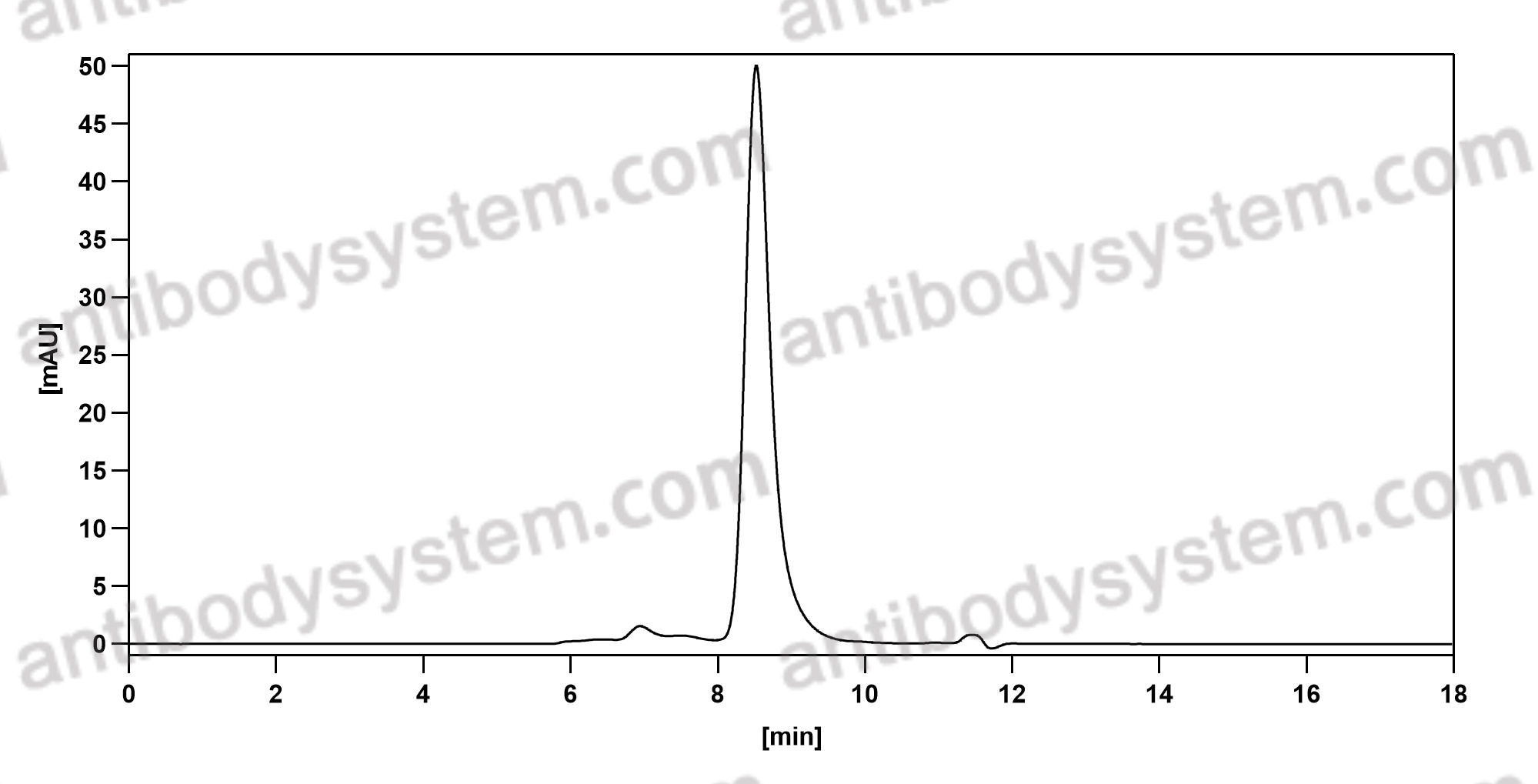
Antibody-drug conjugates of 7-ethyl-10-hydroxycamptothecin: Sacituzumab govitecan and labetuzumab govitecan, PMID: 30822636
Labetuzumab govitecan in metastatic colorectal cancer, PMID: 28844817
Regulation of CEACAM5 and Therapeutic Efficacy of an Anti-CEACAM5-SN38 Antibody-drug Conjugate in Neuroendocrine Prostate Cancer, PMID: 33199493
Phase I/II Trial of Labetuzumab Govitecan (Anti-CEACAM5/SN-38 Antibody-Drug Conjugate) in Patients With Refractory or Relapsing Metastatic Colorectal Cancer, PMID: 28817371
Selective and Concentrated Accretion of SN-38 with a CEACAM5-Targeting Antibody-Drug Conjugate (ADC), Labetuzumab Govitecan (IMMU-130), PMID: 29079710
CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates, PMID: 19789330
Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model, PMID: 28126888
Update of carcinoembryonic antigen radioimmunotherapy with (131)I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group, PMID: 17570017
Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results, PMID: 16170184
A humanized monoclonal antibody to carcinoembryonic antigen, labetuzumab, inhibits tumor growth and sensitizes human medullary thyroid cancer xenografts to dacarbazine chemotherapy, PMID: 15634649
Impact of Different Selectivity between Soluble and Membrane-bound Forms of Carcinoembryonic Antigen (CEA) on the Target-mediated Disposition of Anti-CEA Monoclonal Antibodies, PMID: 31533926
Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft, PMID: 15592930
Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study, PMID: 27763687
Gateways to clinical trials, PMID: 16636723
Development of humanized antibodies as cancer therapeutics, PMID: 15848077
Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug, PMID: 25402018
Gateways to clinical trials, PMID: 15672123
SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody, PMID: 24982436
Multimodal treatment options for bilobar colorectal liver metastases, PMID: 20213463
Clinical-scale radiolabeling of a humanized anticarcinoembryonic antigen monoclonal antibody, hMN-14, with residualizing 131I for use in radioimmunotherapy, PMID: 15632046
A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer, PMID: 15809485
In vitro and in vivo anticancer efficacy of unconjugated humanized anti-CEA monoclonal antibodies, PMID: 18728675
Experimental radioimmunotherapy of small peritoneal metastases of colorectal origin, PMID: 12918078
Biotinylation, pharmacokinetics, and extracorporeal adsorption of humanized MAb 111In-MN14 using an avidin-affinity column in rats, PMID: 12954123
Carcinoembryonic antigen-immunoglobulin Fc fusion protein (CEA-Fc) for identification and activation of anti-CEA immunoglobulin-T-cell receptor-modified T cells, representative of a new class of Ig fusion proteins, PMID: 15002034
Phase I radioimmunotherapy trial with iodine-131--labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer, PMID: 12453334
Prospects of radioimmunotherapy in epithelial ovarian cancer: results with iodine-131-labeled murine and humanized MN-14 anti-carcinoembryonic antigen monoclonal antibodies, PMID: 9441773
Radioimmunotherapy for liver metastases, PMID: 17726786
Initial experience with high-dose radioimmunotherapy of metastatic medullary thyroid cancer using 131I-MN-14 F(ab)2 anti-carcinoembryonic antigen MAb and AHSCR, PMID: 10647610
Phase I/II trial of (131)I-MN-14F(ab)2 anti-carcinoembryonic antigen monoclonal antibody in the treatment of patients with metastatic medullary thyroid carcinoma, PMID: 10223579
Multimodal carcinoembryonic antigen-targeted fluorescence and radio-guided cytoreductive surgery for peritoneal metastases of colorectal origin: single-arm confirmatory trial., PMID:40270484
CEACAM5-Targeted Immuno-PET in Androgen Receptor-Negative Prostate Cancer., PMID:38782457
Multimodal CEA-targeted fluorescence and radioguided cytoreductive surgery for peritoneal metastases of colorectal origin., PMID:35551444
Regulation of CEACAM5 and Therapeutic Efficacy of an Anti-CEACAM5-SN38 Antibody-drug Conjugate in Neuroendocrine Prostate Cancer., PMID:33199493
Impact of Different Selectivity between Soluble and Membrane-bound Forms of Carcinoembryonic Antigen (CEA) on the Target-mediated Disposition of Anti-CEA Monoclonal Antibodies., PMID:31533926
Antibody-drug conjugates of 7-ethyl-10-hydroxycamptothecin: Sacituzumab govitecan and labetuzumab govitecan., PMID:30822636
Selective and Concentrated Accretion of SN-38 with a CEACAM5-Targeting Antibody-Drug Conjugate (ADC), Labetuzumab Govitecan (IMMU-130)., PMID:29079710
Labetuzumab govitecan in metastatic colorectal cancer., PMID:28844817
Phase I/II Trial of Labetuzumab Govitecan (Anti-CEACAM5/SN-38 Antibody-Drug Conjugate) in Patients With Refractory or Relapsing Metastatic Colorectal Cancer., PMID:28817371
Detection of Micrometastases Using SPECT/Fluorescence Dual-Modality Imaging in a CEA-Expressing Tumor Model., PMID:28126888
Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study., PMID:27763687
Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug., PMID:25402018
SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody., PMID:24982436
Multimodal treatment options for bilobar colorectal liver metastases., PMID:20213463
CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates., PMID:19789330
In vitro and in vivo anticancer efficacy of unconjugated humanized anti-CEA monoclonal antibodies., PMID:18728675
Radioimmunotherapy for liver metastases., PMID:17726786
Update of carcinoembryonic antigen radioimmunotherapy with (131)I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group., PMID:17570017
Gateways to clinical trials., PMID:16636723
Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results., PMID:16170184
Development of humanized antibodies as cancer therapeutics., PMID:15848077
A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer., PMID:15809485
Gateways to clinical trials., PMID:15672123
A humanized monoclonal antibody to carcinoembryonic antigen, labetuzumab, inhibits tumor growth and sensitizes human medullary thyroid cancer xenografts to dacarbazine chemotherapy., PMID:15634649
Clinical-scale radiolabeling of a humanized anticarcinoembryonic antigen monoclonal antibody, hMN-14, with residualizing 131I for use in radioimmunotherapy., PMID:15632046
Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft., PMID:15592930
Carcinoembryonic antigen-immunoglobulin Fc fusion protein (CEA-Fc) for identification and activation of anti-CEA immunoglobulin-T-cell receptor-modified T cells, representative of a new class of Ig fusion proteins., PMID:15002034
Biotinylation, pharmacokinetics, and extracorporeal adsorption of humanized MAb 111In-MN14 using an avidin-affinity column in rats., PMID:12954123
Experimental radioimmunotherapy of small peritoneal metastases of colorectal origin., PMID:12918078
Phase I radioimmunotherapy trial with iodine-131--labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer., PMID:12453334
Initial experience with high-dose radioimmunotherapy of metastatic medullary thyroid cancer using 131I-MN-14 F(ab)2 anti-carcinoembryonic antigen MAb and AHSCR., PMID:10647610
Phase I/II trial of (131)I-MN-14F(ab)2 anti-carcinoembryonic antigen monoclonal antibody in the treatment of patients with metastatic medullary thyroid carcinoma., PMID:10223579
Prospects of radioimmunotherapy in epithelial ovarian cancer: results with iodine-131-labeled murine and humanized MN-14 anti-carcinoembryonic antigen monoclonal antibodies., PMID:9441773