Catalog No.
DVV00353
Expression system
Mammalian Cells
Species reactivity
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
Spike glycoprotein, S glycoprotein, E2, Peplomer protein, Spike protein S1, Spike protein S2, Spike protein S2', S, 2
Concentration
2.39 mg/ml
Endotoxin level
Please contact with the lab for this information.
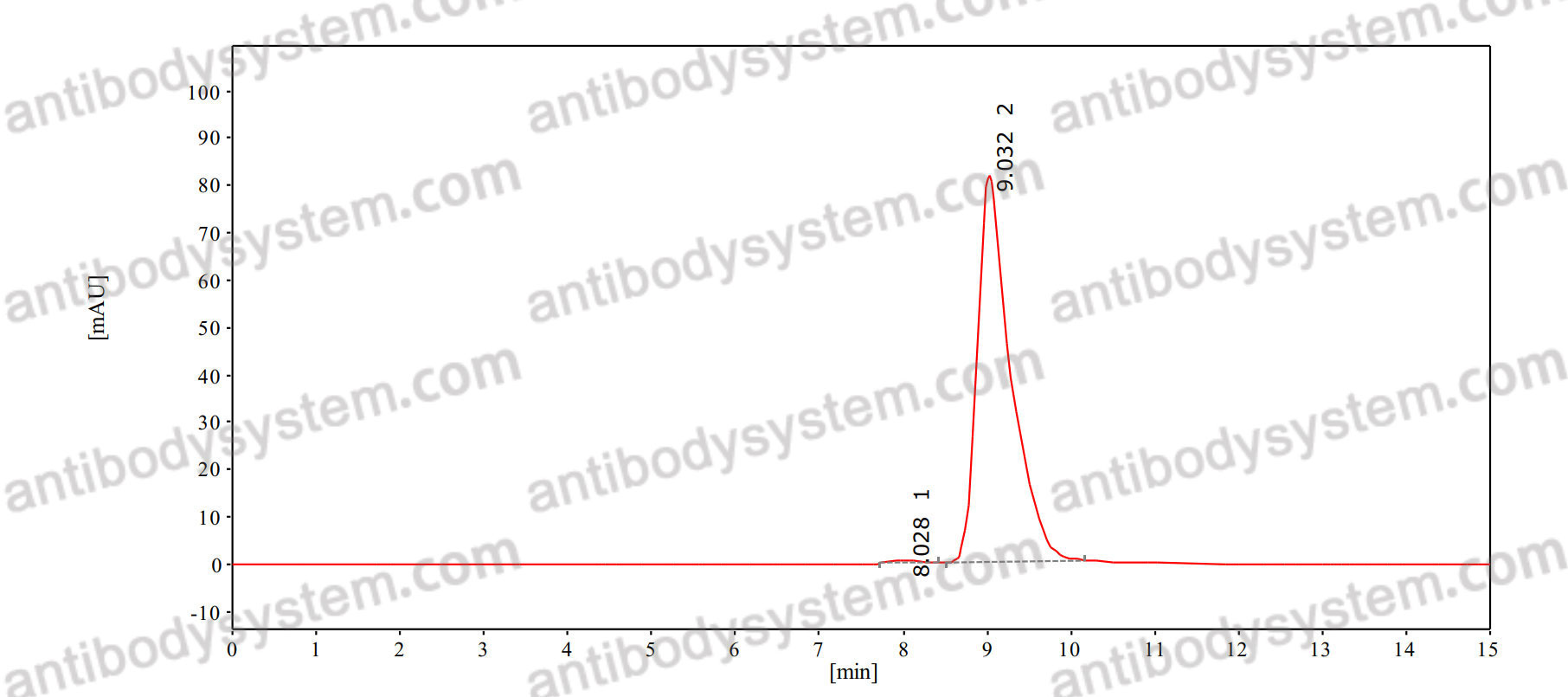
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P0DTC2
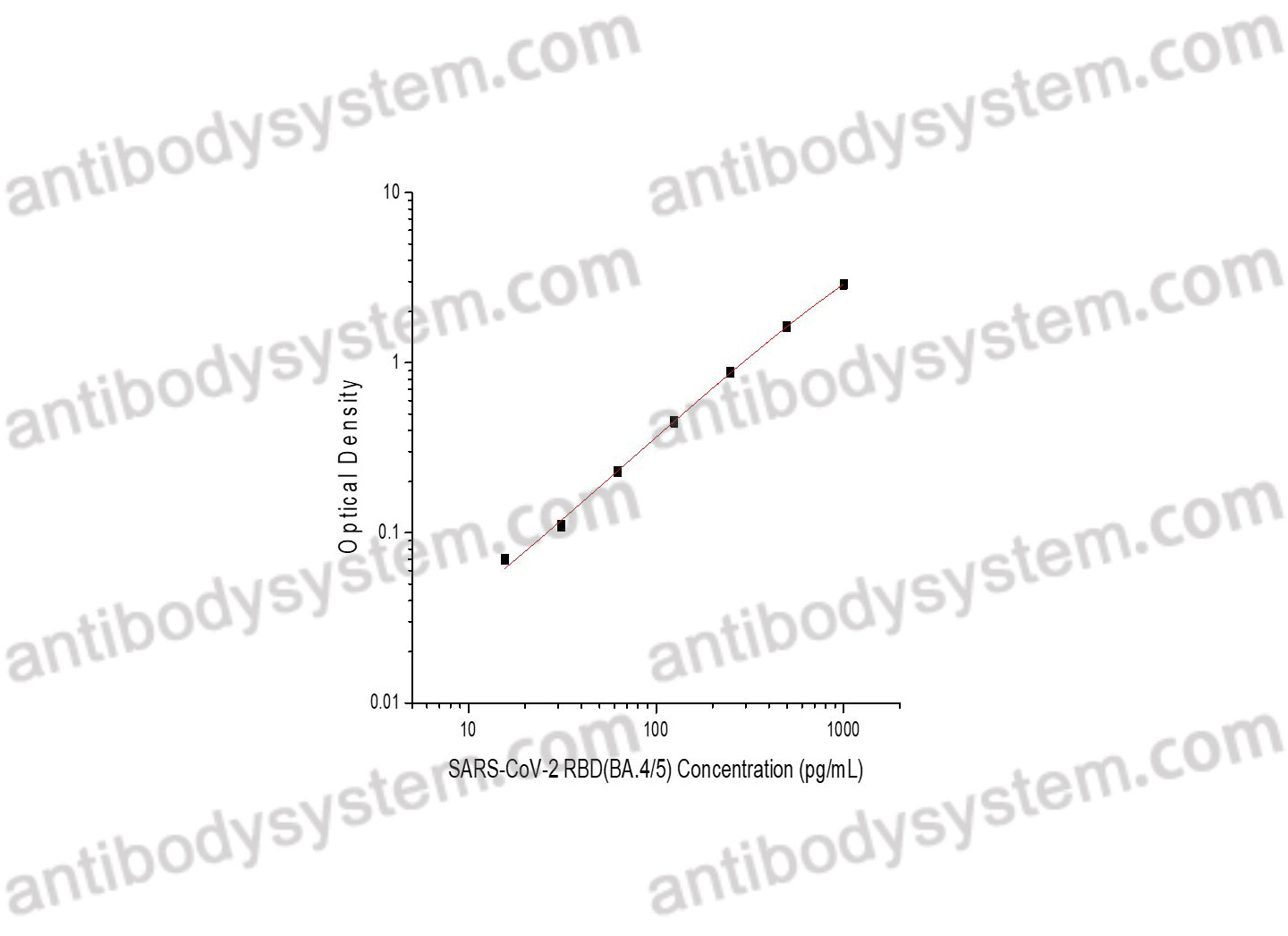
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CAS: 2768288-97-5
Clone ID
Sipavibart
Sipavibart: First Approval., PMID:40488927
Potential use of the SARS-CoV-2 monoclonal antibody sipavibart in people with multiple sclerosis: definition of different patient archetypes from an Italian expert group perspective., PMID:40481170
Dynamics of SARS-CoV-2 variants and mutations in Central Sweden between 2023 and 2024 and their potential implications on monoclonal antibodies pemivibart and sipavibart as PrEP in the region., PMID:40418159
Sipavibart: when a success changes into a failure., PMID:40015293
Efficacy and safety of sipavibart for prevention of COVID-19 in individuals who are immunocompromised (SUPERNOVA): a randomised, controlled, double-blind, phase 3 trial., PMID:40015292
Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP3.3 From Approved Monoclonal Antibodies., PMID:39391808
Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France., PMID:39143811