Catalog No.
KDV00302
Description
PRINCIPLE OF THE ASSAY
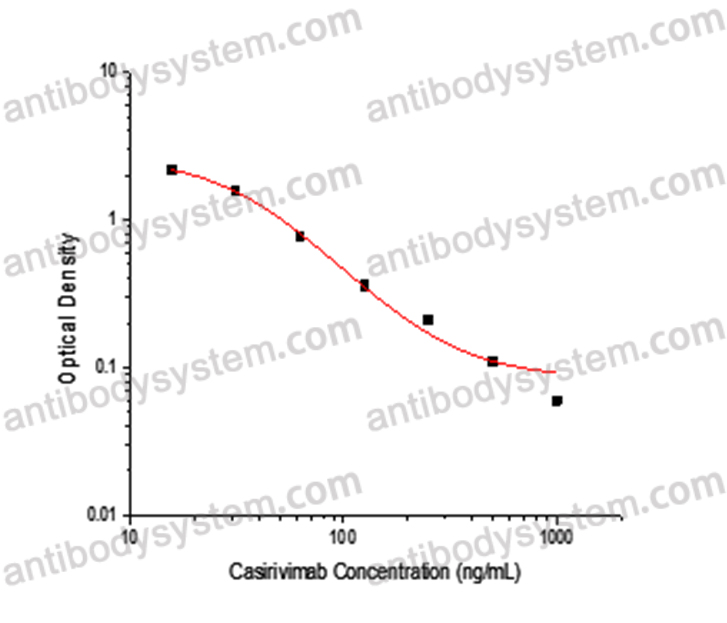
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant SARS-CoV-2 RBD has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Casirivimab in the sample competitively binds to the pre-coated protein with biotin-labeled Casirivimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Casirivimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Casirivimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
15.63 - 1,000 ng/mL
Sensitivity
8.10 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
308.1
|
101.5
|
27.8
|
300.6
|
81.2
|
20.3
|
|
Standard deviation
|
34.6
|
13.8
|
2.7
|
46.1
|
8.2
|
2.6
|
|
CV (%)
|
11.2
|
13.6
|
9.7
|
15.3
|
10.1
|
13.0
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
REGN-10933, REGN10933,CAS: 2415933-42-3
Efficacy and safety of casirivimab/imdevimab (REGEN-COV) in pregnant individuals with COVID-19: Literature review and insights from the COVID-19 International Drug Pregnancy Registry., PMID:40503495
Effectiveness of Anti-SARS-CoV-2 monoclonal antibodies in real-life: RNAemia and clinical outcomes in high-risk COVID-19 patients., PMID:40279347
New Function for Safety Signal Monitoring in MID-NET®: The Case of an Anti-COVID-19 Drug., PMID:40171834
Effectiveness of pharmacological treatments for COVID-19 due to SARS-CoV-2: a systematic literature review., PMID:40093322
Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024., PMID:40084420
Monoclonal Antibodies in Prevention and Early Treatment of COVID-19 in Lung Transplant Recipients: A Systematic Review and Perspective on the Role of Monoclonal Antibodies in the Future., PMID:39995815
Innate and SARS-CoV-2 specific adaptive immune response kinetic in neutralizing monoclonal antibody successfully treated COVID-19 patients., PMID:39832460
Monoclonal antibodies against the spike protein alter the endogenous humoral response to SARS-CoV-2 vaccination and infection., PMID:39504352
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study., PMID:39459877
Assessing the safety and pharmacokinetics of casirivimab and imdevimab (CAS+IMD) in a cohort of pregnant outpatients with COVID-19: results from an adaptive, multicentre, randomised, double-blind, phase 1/2/3 study., PMID:39384241
Safety and Pharmacokinetics of Casirivimab and Imdevimab (CAS + IMD) in Pediatric Outpatients With COVID-19., PMID:39351798
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database., PMID:39345748
Population Pharmacokinetics of Casirivimab and Imdevimab in Pediatric and Adult Non-Infected Individuals, Pediatric and Adult Ambulatory or Hospitalized Patients or Household Contacts of Patients Infected with SARS-COV-2., PMID:39294447
A Retrospective Cohort Observational Study to Assess the Efficacy of Monoclonal Antibody in Coronavirus Disease 2019 Patients., PMID:39291521
Potential immunomodulatory effects of CAS+IMD monoclonal antibody cocktail in hospitalized patients with COVID-19., PMID:39270622
Impact analysis of SARS-CoV-2 vaccination in patients treated with monoclonal antibodies: A monocentric experience., PMID:39265354
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort., PMID:39247344
Antiviral therapy for COVID-19 virus: A narrative review and bibliometric analysis., PMID:39244809
Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial., PMID:39236733
Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities., PMID:39126484
Clinical outcomes in patients with mild to moderate coronavirus disease 2019 treated with monoclonal antibody therapy versus an untreated control cohort., PMID:39066463
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials., PMID:39031183
Safety and Efficacy of SAB-185 for Nonhospitalized Adults With COVID-19: A Randomized Clinical Trial., PMID:39028902
Ronapreve (REGN-CoV; casirivimab and imdevimab) reduces the viral burden and alters the pulmonary response to the SARS-CoV-2 Delta variant (B.1.617.2) in K18-hACE2 mice using an experimental design reflective of a treatment use case., PMID:39012120
A review on the current approaches and perspectives of Covid-19 treatment., PMID:39007473
Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis., PMID:38983147
Prophylactic antibodies inhibit spike-specific T and B cell responses after COVID-19 vaccination., PMID:38965882
Patient-Reported Outcomes in COVID-19 Treatment with Monoclonal Antibodies Reveal Benefits in Return to Usual Activities., PMID:38961047
Unveiling therapeutic dynamics: An in-depth comparative analysis of neutralizing monoclonal antibodies and favipiravir in alleviating COVID-19 outpatients impacts among middle-aged and special populations (MA-FAST)., PMID:38865775
Successful use of tocilizumab and casirivimab/imdevimab in a twin pregnancy with critical COVID-19 - A case report., PMID:38828309
Casirivimab-imdevimab monoclonal antibody treatment for an immunocompromised patient with persistent SARS-CoV-2 infection: a case report., PMID:38824216
Comparison of bamlanivimab with or without etesevimab and casirivimab-imdevimab in clinical outcomes in patients with COVID-19: a systematic review and meta-analysis., PMID:38721976
Treatment of Patients After Lung Transplantation With Covid Infection During Long-Term Follow-Up., PMID:38714369
Viral Kinetics Model of SARS-CoV-2 Infection Informs Drug Discovery, Clinical Dose, and Regimen Selection., PMID:38676291
Development and validation of a prediction score for failure to casirivimab/imdevimab in hospitalized patients with COVID-19 pneumonia., PMID:38529120
Clinical and Virological Outcome of Monoclonal Antibody Therapies Across SARS-CoV-2 Variants in 245 Immunocompromised Patients: A Multicenter Prospective Cohort Study., PMID:38445721
Effectiveness of subcutaneous monoclonal antibody treatment in emergency department outpatients with COVID-19., PMID:38384380
Relapse of COVID-19 and Viral Evolution in a Patient With Good Syndrome: A Case Report., PMID:38371040
Warm autoimmune hemolytic anemia and hemophagocytic lymphohistiocytosis/macrophage activation syndrome occurring after COVID19 infection and administration of Casirivimab + Imdevimab (COVID19 monoclonal antibody)., PMID:38348150
Coronavirus Disease 2019 (COVID-19) in Heart Transplant Recipients and Anti-SARS-CoV-2 Monoclonal Antibodies: Experience, Lessons Learnt, and Future Challenges., PMID:38334977
Plant-derived compounds as potential leads for new drug development targeting COVID-19., PMID:38281731
Real-world prescription of anti-COVID-19 drugs in hospitalized patients with COVID-19 in Japan., PMID:38277429
Clinical Efficacy of Imdevimab/Casirivimab for Persistent Omicron SARS-CoV-2 Infection in Patients with Hematological Malignancies., PMID:38171874
[Use of a combination of the virus-neutralizing monoclonal antibodies casirivimab and imdevimab for mild to moderate COVID-19 in patients at high risk of progression: Results of the non-interventional observational study]., PMID:38158969
The II Brazilian Guidelines for the pharmacological treatment of patients hospitalized with COVID-19 Joint Guidelines of the Associação Brasileira de Medicina de Emergência, Associação de Medicina Intensiva Brasileira, Associação Médica Brasileira, Sociedade Brasileira de Angiologia e Cirurgia Vascular, Sociedade Brasileira de Infectologia, Sociedade Brasileira de Pneumologia e Tisiologia and Sociedade Brasileira de Reumatologia., PMID:38133154
Nasopharyngeal Viral Load Is the Major Driver of Incident Antibody Immune Response to SARS-CoV-2 Infection., PMID:38111750
Neutralizing monoclonal antibodies for the prevention of severe COVID-19: a retrospective study during Omicron BA.1 variant surge., PMID:38095569
The proteolytic airway environment associated with pneumonia acts as a barrier for treatment with anti-infective antibodies., PMID:38086491
Randomized trial of the safety and efficacy of anti-SARS-CoV-2 mAb in the treatment of patients with nosocomial COVID-19 (CATCO-NOS)., PMID:38058498
Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial., PMID:38058433