Catalog No.
KDV00301
Description
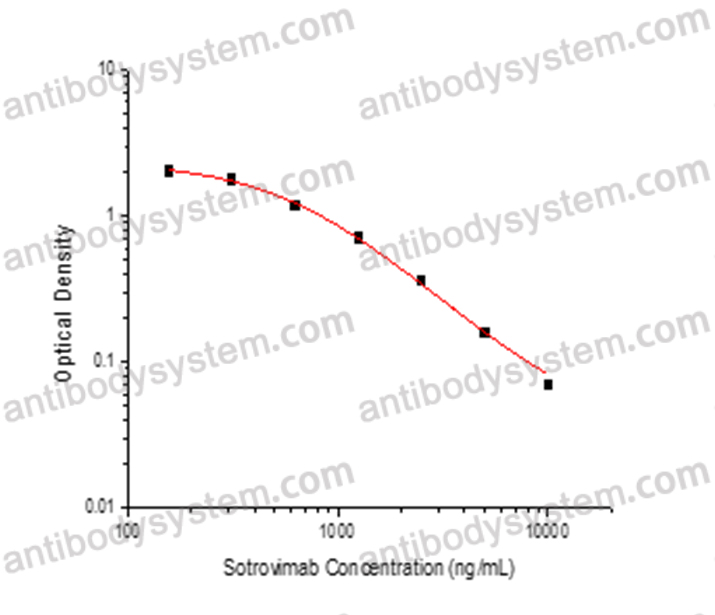
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant SARS-CoV-2 RBD has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Sotrovimab in the sample competitively binds to the pre-coated protein with biotin-labeled Sotrovimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Sotrovimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Sotrovimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 ng/mL - 10,000 ng/mL
Sensitivity
30.51 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
3831.47
|
1199.88
|
314.02
|
4124.27
|
1082.39
|
380.26
|
|
Standard deviation
|
268.61
|
52.47
|
44.62
|
618.41
|
204.23
|
75.62
|
|
CV (%)
|
7.01
|
4.37
|
14.21
|
14.99
|
18.87
|
19.89
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
VIR-7831,GSK4182136,S309,GSK-4182136,CAS: 2423014-07-5
Monitoring the emergence of resistance with sotrovimab in immunocompromised patients with COVID-19: LUNAR study., PMID:40398499
An integrated multiscale computational framework deciphers SARS-CoV-2 resistance to sotrovimab., PMID:40394898
Case Report: Persistent COVID-19 in a fully vaccinated Japanese man being treated with rituximab and epcoritamab for diffuse large B-cell lymphoma., PMID:40370717
Comparative Analysis of Early COVID-19 Treatment Efficacy in a Multicentric Regional Cohort in Italy: Emulation of a Series of Target Trials., PMID:40326978
Antiviral prescription of mild to moderate COVID-19 and adherence to official recommendations in Spanish emergency departments., PMID:40320950
Effectiveness of Anti-SARS-CoV-2 monoclonal antibodies in real-life: RNAemia and clinical outcomes in high-risk COVID-19 patients., PMID:40279347
Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients., PMID:40160532
Pharmacokinetics and Safety of Single-Dose Sotrovimab in High-Risk Children and Adolescents With Mild-to-Moderate COVID-19., PMID:40146813
Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data., PMID:40142695
Efficacy and Safety of Sotrovimab Versus Oral Antiviral for Early Treatment in High-Risk Patients in Omicron Era: A Multicenter Retrospective Study., PMID:40137701
Impact of treatment of COVID-19 with sotrovimab on post-acute sequelae of COVID-19 (PASC): an analysis of National COVID Cohort Collaborative (N3C) data., PMID:40120069
Effectiveness of pharmacological treatments for COVID-19 due to SARS-CoV-2: a systematic literature review., PMID:40093322
Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024., PMID:40084420
SARS-CoV-2 genomic evolution during a severe and long-lasting omicron infection under antiviral therapy., PMID:40082784
Accurate evaluation of live-virus microneutralisation for SARS-CoV-2 variant JN.1 in the assessment of vaccination and therapeutics., PMID:40056806
Monoclonal Antibodies in Prevention and Early Treatment of COVID-19 in Lung Transplant Recipients: A Systematic Review and Perspective on the Role of Monoclonal Antibodies in the Future., PMID:39995815
Real-World Effectiveness of Sotrovimab in Patients Infected With SARS-CoV-2 Omicron Subvariant BA.2 in Western Sydney, Australia., PMID:39945377
Long-term outcomes of passive immunotherapy for COVID-19: a pooled analysis of a large multinational platform randomized clinical trial., PMID:39922466
Real-World Effectiveness of Sotrovimab in Ambulatory Patients With COVID-19: A Retrospective Cohort Study Using a Large Administrative Claims Database in the United States., PMID:39909767
Safety and tolerability of intramuscular sotrovimab administered at different injection sites: results from the Phase 1 COSMIC study., PMID:39881564
The evolving landscape of COVID-19: factors associated with in-hospital COVID-19 related mortality during the 2023-2024 phase of JN.1 subvariant dominance., PMID:39871210
Fc-binding nanodisc restores antiviral efficacy of antibodies with reduced neutralizing effects against evolving SARS-CoV-2 variants., PMID:39856746
Uptake and safety of Sotrovimab for prevention of severe COVID-19 in a cohort and self-controlled case series study., PMID:39820075
Comparative Effectiveness of Outpatient COVID-19 Therapies in Solid Organ Transplant Recipients., PMID:39791933
Affinity maturation endows potent activity onto class 6 SARS-CoV-2 broadly neutralizing antibodies., PMID:39746041
Comparative Effectiveness of Antivirals and Monoclonal Antibodies for Treating COVID-19 Patients Infected With Omicron Variant: A Systematic Review and Network Meta-Analysis., PMID:39722466
Pharmacokinetics of the Monoclonal Antibody, Sotrovimab, in Healthy Participants Following IM Administration at Different Injection Sites., PMID:39668507
Early combination of sotrovimab with nirmatrelvir/ritonavir or remdesivir is associated with low rate of persisting SARS CoV-2 infection in immunocompromised outpatients with mild-to-moderate COVID-19: a prospective single-centre study., PMID:39661366
Therapeutic Potential of Neutralizing Monoclonal Antibodies (nMAbs) against SARS-CoV-2 Omicron Variant., PMID:39543801
Characterisation of the antibody-mediated selective pressure driving intra-host evolution of SARS-CoV-2 in prolonged infection., PMID:39405332
Comparison of the Neutralization Power of Sotrovimab Against SARS-CoV-2 Variants: Development of a Rapid Computational Method., PMID:39388246
Real-world effectiveness of early anti-SARS therapy in severely immunocompromised COVID-19 outpatients during the SARS-CoV-2 omicron variant era: a propensity score-adjusted retrospective cohort study., PMID:39374379
Does early combination vs. Monotherapy improve clinical outcomes of clinically extremely vulnerable patients with COVID-19? Results from a retrospective propensity-weighted analysis., PMID:39367485
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database., PMID:39345748
Pooled analysis of the MANTICO2 and MONET randomized controlled trials comparing drug efficacy for early treatment of COVID-19 during Omicron waves., PMID:39343244
Identification of antibody-resistant SARS-CoV-2 mutants via N4-Hydroxycytidine mutagenesis., PMID:39293594
Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial., PMID:39265595
Impact analysis of SARS-CoV-2 vaccination in patients treated with monoclonal antibodies: A monocentric experience., PMID:39265354
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort., PMID:39247344
Antiviral therapy for COVID-19 virus: A narrative review and bibliometric analysis., PMID:39244809
Sotrovimab lost neutralization efficacy against SARS-CoV-2 subvariants but remained clinically effective: Were monoclonal antibodies against COVID-19 rejected too early?, PMID:39137507
Monoclonal Antibody Therapies Against SARS-CoV-2: Promises and Realities., PMID:39126484
In Vitro and In Vivo Neutralizing Efficacy of Monoclonal Antibodies Against Sars-Cov-2 Variants in Kidney Transplant Recipients., PMID:39081903
Characteristics and outcomes of patients treated with sotrovimab to prevent progression to severe COVID-19 in Belgium., PMID:39081095
Saliva Is a Sensitive and Accessible Sample Both for SARS-CoV-2 Detection and for the Evaluation of Treatment Effectiveness in Follow-Up Studies., PMID:39066203
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials., PMID:39031183
The safety of antivirals and neutralising monoclonal antibodies used in prehospital treatment of Covid-19., PMID:39019401
Real-world effectiveness of sotrovimab in preventing hospitalization and mortality in high-risk patients with COVID-19 in the United States: A cohort study from the Mayo Clinic electronic health records., PMID:39012863
A review on the current approaches and perspectives of Covid-19 treatment., PMID:39007473