Catalog No.
KDD12605
Description
PRINCIPLE OF THE ASSAY
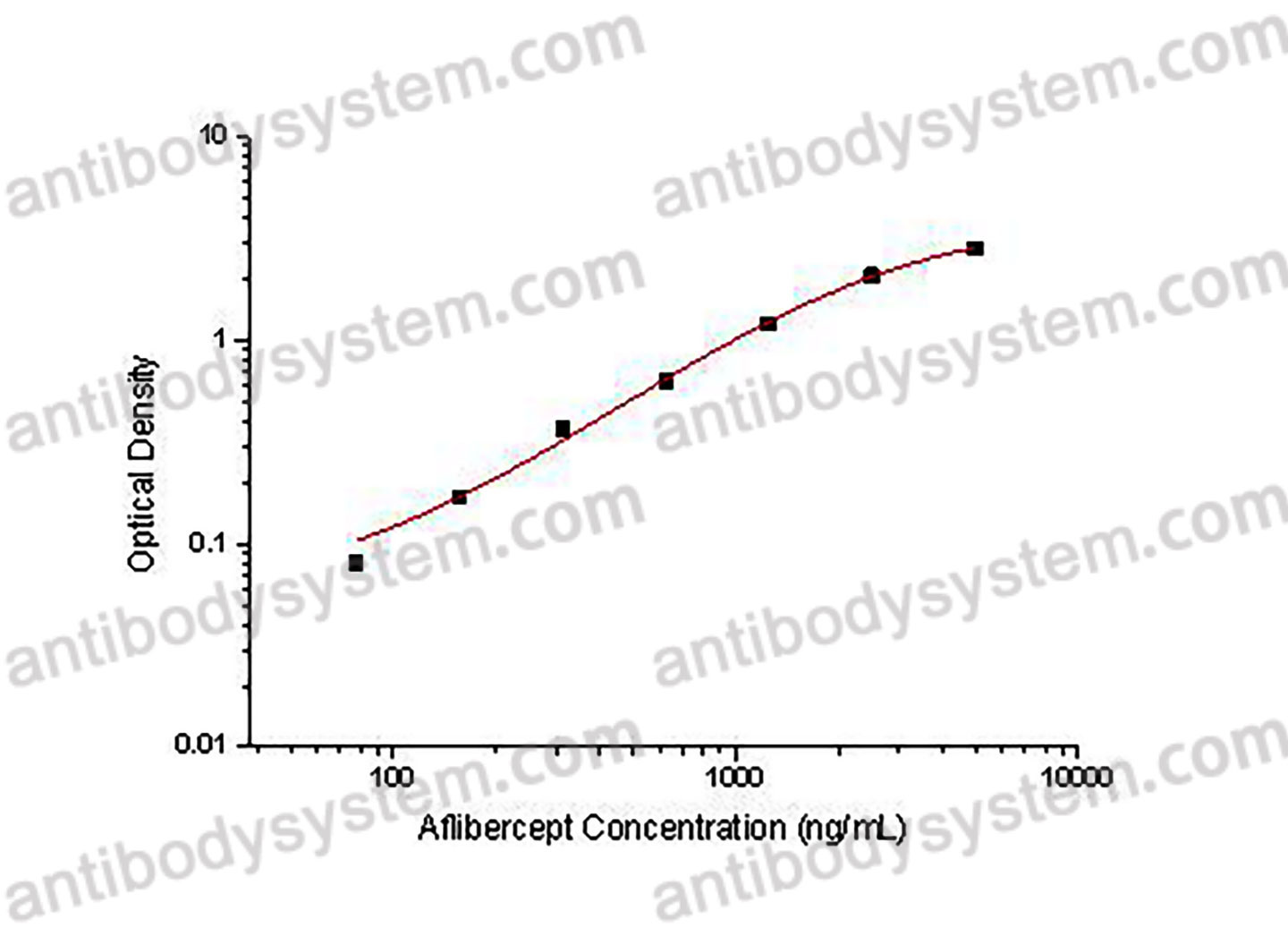
This assay employs the quantitative sandwich enzyme immunoassay technique. An antibody specific for Aflibercept has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Aflibercept present is bound by the immobilized antibody. After washing away any unbound substances, a biotin-labeled antibody specific for Aflibercept is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Aflibercept bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Aflibercept concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
78.13 - 5,000 ng/mL
Sensitivity
40.12 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2226.0
|
599.3
|
139.1
|
2254.9
|
651.9
|
149.7
|
|
Standard deviation
|
139.0
|
34.0
|
13.8
|
193.3
|
86.5
|
21.4
|
|
CV (%)
|
6.2
|
5.7
|
9.9
|
8.6
|
13.3
|
14.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
CAS: 862111-32-8
A randomized, double-masked parallel-group, multicenter clinical study evaluating the efficacy and safety of the biosimilar candidate AVT06 compared to the reference product aflibercept in participants with neovascular age-related macular degeneration., PMID:40512025
Six-year findings of polypoidal choroidal vasculopathy in the EVEREST II study: Japanese subgroup analysis., PMID:40504425
Factors determining timing of first recurrence after three loading aflibercept injections in newly diagnosed neovascular age-related macular degeneration., PMID:40504424
Efficacy of switching from existing anti-vascular endothelial growth factor drugs to ranibizumab biosimilar in neovascular age-related macular degeneration., PMID:40500585
A Prospective Observational Study of Intravitreal Aflibercept in Retinal Vein Occlusion with Initially Good Visual Acuity., PMID:40499162
Effect of Faricimab versus Aflibercept on Hyperreflective Foci in Patients with Diabetic Macular Edema from the YOSEMITE/RHINE Trials., PMID:40496217
Feedback regulation between histone lactylation and ALKBH3-mediated glycolysis regulates age-related macular degeneration pathology., PMID:40493193
Usage of brolucizumab as treatment for wet age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV): A narrative review., PMID:40489855
Anti-VEGFs for Diabetic Macular Oedema: Analysis of Efficacy, Safety, and Cost of More Durable Therapies from a Dutch Societal Perspective., PMID:40481910
Intravitreal anti-vascular endothelial growth factor injections and risks of stroke in patients with neovascular age-related macular degeneration-A registry-based cohort study., PMID:40481786
A swept-source optical coherence tomography study of the spectrum of laser pointer maculopathy., PMID:40474312
TALON Phase IIIb Study: 32 Week Primary Results of Brolucizumab Using Treat and Extend for Neovascular Age Related Macular Degeneration., PMID:40466770
Corrigendum: Efficacy and safety of intravitreal injection of aflibercept biosimilar for treating diabetic macular edema., PMID:40458651
Extended duration of VEGF inhibition with aflibercept 8 mg: the role of reduced ocular clearance., PMID:40456958
Author Correction: Effects of switching from intravitreal injection of aflibercept to faricimab on ocular blood flow in patients with diabetic macular edema., PMID:40456888
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Anti-VEGF Agents Clearance Through the Aqueous Outflow Pathway in a Rat Model., PMID:40455045
Deep learning-assisted analysis of biomarker changes after increase of dosing from aflibercept 2 mg to 8 mg in therapy-resistant neovascular age-related macular degeneration., PMID:40451292
Managing Choroidal Neovascularization in Pseudoxanthoma Elasticum: Outcomes of Vitrectomy and Intravitreal Ranibizumab/Aflibercept Therapy-A Case Report., PMID:40438152
Oral lamivudine in diabetic macular edema: A randomized, double-blind, placebo-controlled clinical trial., PMID:40436012
Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration., PMID:40423060
Effectiveness and safety of anti-vascular endothelial growth factor therapies for macular edema in retinal vein occlusion: A systematic review and network meta-analysis of randomized controlled trials., PMID:40419166
Successful Response to Intravitreal Faricimab Injections in a Case of Neovascular Age-Related Macular Degeneration in Vitrectomized Eyes., PMID:40400877
Twelve-Month Outcomes of Faricimab for Patients with Sub-Optimally Responsive Diabetic Macular Oedema: A Retrospective Single-Centre Study., PMID:40396158
Response: Aflibercept for the treatment of pigmentary retinopathy in Kearns-Sayre Syndrome?, PMID:40392487
Aflibercept for the treatment of pigmentary retinopathy in Kearns-Sayre syndrome?, PMID:40389721
Socioeconomic Disparities in Intravitreal Injection Use and Anti-VEGF Agent Selection: Aflibercept/Ranibizumab Versus Bevacizumab., PMID:40383690
Prospective evaluation of soluble CD14 as a biomarker following five aflibercept treatments in diabetic macular edema., PMID:40379891
Successful use of aflibercept for high cardiac output in hereditary hemorrhagic telangiectasia after failure of pazopanib and bevacizumab therapy., PMID:40379104
Recurrence of Macular Edema in Branch Retinal Vein Occlusion-A Comparison of Aflibercept and Ranibizumab in a Randomized Trial., PMID:40373873
Sustainable Practices in Anti-VEGF Therapy: A 15-Year Bibliometric Analysis of Ranibizumab for Age-Related Macular Degeneration., PMID:40365539
Faricimab Reverts VEGF-A165-Induced Impairment of the Barrier Formed by Retinal Endothelial Cells., PMID:40362554
Silicone oil microdroplets from syringes with intravitreal anti-vascular endothelial growth factor and complement inhibitor injections., PMID:40359347
Bilateral Idiopathic Multifocal Pigment Epithelial Detachments: A Case Series and Review of Literature., PMID:40359333
Visual outcome following initiation of first injection versus after three monthly doses of aflibercept 2 mg for treatment naïve age-related macular degeneration to inform clinical trial designs: PRECISE Report No. 6., PMID:40355704
Long-term outcomes of the observe-and-plan regimen in treating neovascular age-related macular degeneration: a retrospective real-life analysis., PMID:40348918
Comprehensive multi-omics and pharmacokinetics reveal sclareol's role in inhibiting ocular neovascularization., PMID:40347925
Anti-VEGF drugs compared with laser photocoagulation for the treatment of diabetic retinopathy: a systematic review and economic analysis., PMID:40347224
Plain-language commentary: 2-year findings from the FIREFLEYE next study looking at how well aflibercept injected into affected eyes in babies with retinopathy of prematurity works and how safe it is compared with laser treatment., PMID:40342575
Improved functional and morphological outcomes with faricimab in nAMD eyes with poor response to prior intravitreal anti-VEGF therapy., PMID:40338383
Endophthalmitis following intravitreal injections at a tertiary center: real-life data on incidence, risk factors, management strategies, and visual outcomes., PMID:40335933
Uncommon Therapeutic Approaches for Patients with Recurrent Central Serous Chorioretinopathy: case report and literature review., PMID:40330964
Different clinical presentations of persistent placoid maculopathy: a case series., PMID:40329229
Comparison of treatment routine using aflibercept: Strict vs. relaxed retreatment regimen (TOLERANT study)-A non-inferiority, randomized controlled trial., PMID:40326420
Tailored Anti-VEGF Therapy with New Generation Optimizations (TANGO) Treatment Regimen for Neovascular Age-Related Macular Degeneration: Rationale, Design, and Simulation Study., PMID:40321164
Aflibercept 2 mg biosimilar (Tyalia)-real-world experience from IRAN (ATRIA study)., PMID:40319174
Acute, not delayed, treatment of aflibercept enhances vessel density in post-ischemic brain and promotes long-term stroke recovery in obese mice., PMID:40314397
Noninfectious Intraocular Inflammation After Intravitreal Aflibercept., PMID:40310633
EN FACE DEPTH-RESOLVED OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY MONITORING OF MACULAR OUTER RETINAL DEPOSITS IN A CASE OF MULTIFOCAL CHOROIDITIS MIMICKING SYPHILITIC UVEITIS., PMID:40299547
Real-world safety and efficacy of Anti-VEGF treatment in Brazil., PMID:40298749


 ELISA Kit -antibodysystem.jpg)