Catalog No.
KAB94401
Description
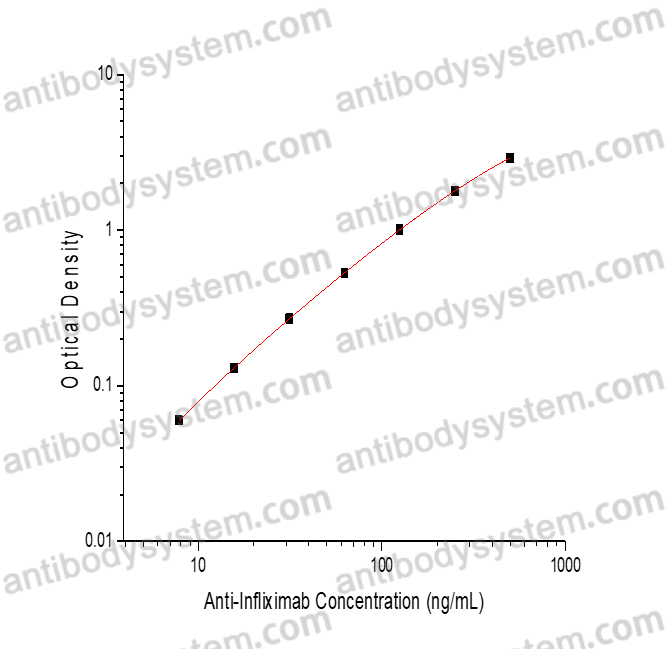
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Adalimumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and anti-Adalimumab will be captured by immobilized Adalimumab. After washing away any unbound substances, a biotin-labeled Adalimumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Adalimumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Adalimumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.81 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
46.5
|
11.0
|
2.8
|
50.2
|
12.4
|
3.0
|
|
Standard deviation
|
1.8
|
0.9
|
0.3
|
7.2
|
0.7
|
0.3
|
|
CV (%)
|
3.9
|
8.4
|
10.1
|
14.3
|
5.4
|
10.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ABP 501,D2E7, Adalimumab,CAS: 331731-18-1
Therapeutic Potential of Upadacitinib in Adolescents with Idiopathic Chronic Panuveitis Who Lost Response to Adalimumab., PMID:40515519
Association between rapid and sustained remission and clinician- and patient-reported outcomes in patients with rheumatoid arthritis: post hoc analysis of data from the SELECT-COMPARE study., PMID:40514717
Retinal venous occlusion in drug-induced lupus., PMID:40514329
Long-Term Effectiveness and Safety of Weekly Adalimumab in Refractory Non-Infectious Uveitis., PMID:40511491
A physiologically based pharmacokinetic model to describe [89Zr]Zr-DFO-Adalimumab specific activity after intravitreal administration to rats with endotoxin-induced uveitis., PMID:40505897
Development and validation of a novel Endoscopic ulcer Activity Score for Evaluation of Crohn's Disease (EASE-CD) using data from two randomised controlled trials., PMID:40505666
Summary of Research: Immunogenicity of Adalimumab Reference Product and Adalimumab-adbm in Patients with Rheumatoid Arthritis, Crohn's Disease, and Chronic Plaque Psoriasis: A Pooled Analysis of the VOLTAIRE trials., PMID:40498294
Upadacitinib for ulcerative colitis and pyoderma gangrenosum in a patient with schizophrenia on long-term risperidone: A case report., PMID:40495949
Efficacy and Safety of ABBV-154 for the Treatment of Active Rheumatoid Arthritis: A Phase 2b, Randomized, Placebo-Controlled Trial., PMID:40495262
Deep learning-based ranking method for subgroup and predictive biomarker identification in patients., PMID:40494908
A single-cell RNA-seq dataset of synovial fluid from rheumatoid arthritis treated with TNF-α/JAK inhibitor., PMID:40494845
Comparison and analysis of biological drug consumption in two Italian hospital settings: Governance actions and prescribing appropriateness., PMID:40494065
Multiple Switches Between Adalimumab-fkjp and Reference Adalimumab in Moderate-to-Severe Chronic Plaque Psoriasis: A Multicenter, Double-Blind, Parallel Group, Randomized Clinical Trial for Interchangeability., PMID:40493334
Management of Pediatric Acne Vulgaris and Hidradenitis Suppurativa in Minoritized and Underserved Populations., PMID:40489363
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Reply to Correspondence to Recurrence Risk in Pediatric Non-Infectious Uveitis during Adalimumab Tapering., PMID:40485634
Drug survival, long-term safety and efficacy of anti-TNF-alpha biosimilars: a monocentric retrospective study., PMID:40485573
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Tumor necrosis factor α inhibitor-induced alopecia in pediatric patients: a cohort of 20 patients and review of the literature., PMID:40481366
A Survey of Diagnostic and Management Practices in Retinal Vasculitis: International Uveitis Study Group (IUSG) Retinal Vasculitis Study (ReViSe)-Report 5., PMID:40478525
Effects of Adalimumab on Mitochondria of Psoriatic Mesenchymal Stem Cells., PMID:40478194
Case Report: Kyrieleis plaques in an unusual Behcet's disease uveitis., PMID:40475959
Case Report: Effectiveness of deucravacitinib in chronic recurrent multifocal osteomyelitis and concomitant psoriasis., PMID:40475785
Pharmacotherapy for non-infectious uveitis: spotlight on phase III clinical trials of locally injected or implanted therapeutics and systemic immunomodulatory drugs., PMID:40473986
Anti-tumor Necrosis Factor-Alpha (TNF-α)-Induced Hypertrophic Lichen Planus: A Case Report., PMID:40470457
Pharmacogenomics of TNF inhibitors., PMID:40469293
Lupus panniculitis secondary to treatment with adalimumab in a patient with rheumatoid arthritis., PMID:40466251
Adalimumab Monotherapy vs Adalimumab With Methotrexate for Psoriasis., PMID:40465280
High-Throughput Human Gut Immune Co-Culture Model for Evaluating Inflammatory Bowel Disease Anti-Inflammatory Therapies., PMID:40462955
Benefit-risk analysis of upadacitinib versus adalimumab in patients with rheumatoid arthritis and higher or lower risk of cardiovascular disease., PMID:40461266
Severe paradoxical generalized pustular psoriasis induced by adalimumab biosimilar successfully treated with brodalumab., PMID:40457932
Correspondence to Recurrence Risk in Pediatric Non-Infectious Uveitis during Adalimumab Tapering: An International Multicenter Retrospective Study., PMID:40456698
Treatment of Autoimmune Enteropathy With Vedolizumab., PMID:40452653
Immunoglobulin A vasculitis and pustular psoriasis precipitated by Tawon Liar: a case report., PMID:40448236
Letter to the Editor: Assessing early therapeutic drug monitoring of adalimumab as a predictor of treatment efficacy and immunogenicity in rheumatic diseases: "early therapeutic drug monitoring of adalimumab"., PMID:40445523
Clinical Response to Adalimumab Therapy and Its Determinants in Patients With Radiographic Axial Spondyloarthritis: A Prospective Real-World Study in Taiwan., PMID:40443026
Cross-phenotype genome-wide association study supports shared genetic etiology between skin and gastrointestinal tract diseases., PMID:40441863
Physician Burden and Time Delays in Initiating Immunomodulatory Therapy for Non-infectious Uveitis and Inflammatory Eye Diseases., PMID:40441503
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Uveitis in Adults: A Review., PMID:40434762
Failure of primary immunosuppressive agents in uveitis., PMID:40434459
JAK-STAT inhibitors in noninfectious uveitis - A review., PMID:40434456
Optimizing Biologic Therapy for the Prevention of Post-Operative Recurrence in Crohn's Disease: Current Evidence and Future Perspectives., PMID:40427059
Subconjunctival Adalimumab for Noninfectious Uveitis: A Prospective Pilot Study., PMID:40423087
Anti-Adalimumab Antibodies Purified from Juvenile Idiopathic Arthritis Patients: Kinetic Characterization Among Biosimilars., PMID:40422017
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Comparative efficacy, safety and immunogenicity of biosimilars and their reference biologic drugs in ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40418343