Catalog No.
KAC36901
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Tocilizumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and anti-Tocilizumab will be captured by immobilized Tocilizumab. After washing away any unbound substances, a biotin-labeled Tocilizumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Tocilizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
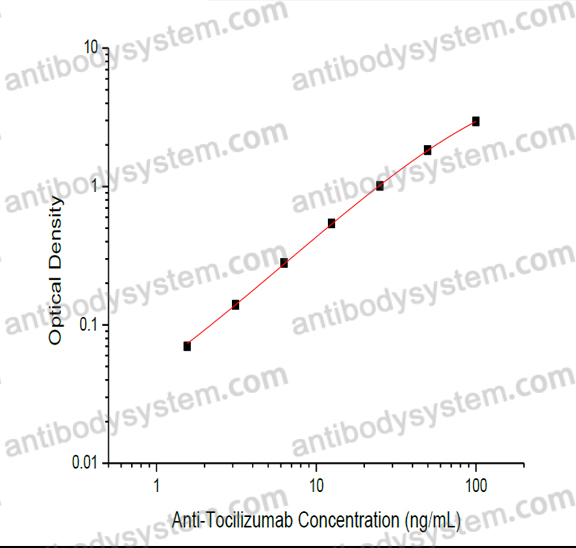
Range
1.56 - 100 ng/mL
Sensitivity
0.81 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
46.0
|
10.9
|
2.1
|
41.2
|
11.9
|
2.7
|
|
Standard deviation
|
1.6
|
0.3
|
0.2
|
3.8
|
1.4
|
0.3
|
|
CV (%)
|
3.5
|
3.2
|
9.3
|
9.3
|
12.0
|
10.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
Atlizumab, MRA / R-1569 / RHPM-1 / RO-4877533,CAS: 375823-41-9
Hemoadsorption as a Supportive Strategy for Severe Toxicity Associated With Chimeric Antigen Receptor T-Cell Therapy: A Case Series., PMID:40510619
Key indicators for guiding tocilizumab therapy to prevent orbital decompression surgery in hormone-resistant dysthyroid optic neuropathy., PMID:40510358
The clinical toxicity of advanced therapy medicinal products., PMID:40509780
Sarilumab in the treatment of rheumatoid arthritis: future perspectives., PMID:40506803
Pulmonary Aspergillosis: Epidemiology and unresolved diagnostic challenges - insights from a two-year retrospective cohort study in Marseille., PMID:40505837
Unravelling VEXAS syndrome: shedding light on a recently recognised medical condition., PMID:40499953
A rare case report: acute necrotizing encephalopathy and acute fulminant myocarditis., PMID:40495992
Clinical characteristics and treatment of infantile Takayasu arteritis in the Chinese Han population: a single-center study., PMID:40495185
Therapeutic Choices in Systemic Sclerosis-Associated Interstitial Lung Disease, a Survey of 2 International Research Groups., PMID:40489954
Management of Patients With COVID-19 During the Pandemic: A Retrospective Cohort Study From a Tertiary Care Centre in Mumbai, India., PMID:40486438
Minocycline in chronic management of febrile infection-related epilepsy syndrome (FIRES): a case series and literature review of treatment strategies., PMID:40481521
Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial., PMID:40480650
Tumefactive demyelination as the first presentation of MOG ab-associated disease., PMID:40479755
Efficacy of tocilizumab monotherapy after ultrashort glucocorticoid administration to treat giant cell arteritis: three year follow-up., PMID:40478774
[VEXAS Syndrome]., PMID:40476413
Steroid refractory immune-related acute disseminated encephalomyelitis (ADEM) successfully treated with 2nd and 3rd line immunosuppressive therapy., PMID:40473324
[Clinical phenotype and genotype analysis of neuroinflammation, autoinflammation, splenomegaly and anemia syndrome caused by IRAK4 gene variant]., PMID:40468499
Belatacept in Kidney Transplantation: Reflecting on the Past, Shaping the Future., PMID:40463417
[Sequential treatment with siltuximab and tocilizumab for childhood idiopathic multicentric Castleman disease: a case report]., PMID:40462437
Baricitinib and Tocilizumab in COVID-19: A Call for Deeper Mechanistic and Methodological Exploration., PMID:40459395
IL-6R blockade combined with immunosuppressants alleviates adult-onset Still's disease through immune remodeling: a mass cytometry study., PMID:40457438
Tocilizumab-Based Treatment of Microvascular Inflammation in Kidney Transplant Recipients: A Retrospective Study., PMID:40454296
The first nationwide epidemiological survey of chronic recurrent multifocal osteomyelitis in Japan., PMID:40445191
Physician Burden and Time Delays in Initiating Immunomodulatory Therapy for Non-infectious Uveitis and Inflammatory Eye Diseases., PMID:40441503
Antibody-mediated Rejection - Treatment Standard., PMID:40440205
Mendelian randomisation and infection: pitfalls and promises., PMID:40435229
Successful treatment with tocilizumab in a case of familial Mediterranean fever with Takayasu arteritis., PMID:40433865
Use of Immune Modulating Agents to Regulate Hyperinflammation in Severe COVID 19: Assessment of Tocilizumab Use in Combination with Steroids., PMID:40432839
Efficacy of Tofacitinib in Takayasu Arteritis Refractory to Biologic DMARDs-A Multicentre Study in Indian Patients., PMID:40432338
New Challenging Systemic Therapies for Juvenile Scleroderma: A Comprehensive Review., PMID:40430462
Experience of High Tibial Osteotomy for Patients with Rheumatoid Arthritis Treated with Recent Medication: A Case Series., PMID:40429328
Interactions of Probiotics with the Immune Cells of Patients with COVID-19 Pneumonia., PMID:40424990
Tocilizumab prophylaxis in double cord blood transplantation., PMID:40423984
Phase 2 trial of cyclosporine-A, mycophenolate mofetil, and tocilizumab GVHD prophylaxis in cord blood transplantation., PMID:40423982
Polyangiitis overlap syndrome is a high-risk clinical phenotype for relapse: case series from the KEIO-vasculitis cohort., PMID:40421732
Anti-MDA5 antibody positive dermatomyositis complicated by interstitial lung disease with an improved outcome: A case report., PMID:40419927
Tocilizumab effectiveness in paediatric non-infectious uveitis: data from the International AIDA Network Registries on ocular inflammatory disorders., PMID:40413020
Treatment outcomes in patients with VEXAS syndrome: a retrospective cohort study., PMID:40412417
Integrative genome-wide association studies, proteome-wide Mendelian randomization, and single-cell RNA sequencing identify interleukin-6 receptor protein as a therapeutic target in aortic aneurysm., PMID:40409638
Management of immune-related myocarditis, myositis and myasthenia gravis (MMM) overlap syndrome: a single institution case series and literature review., PMID:40406130
Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves' orbitopathy: a systematic review and meta-analysis., PMID:40404973
[Update of clinical management in autoimmune encephalitis-2004]., PMID:40399061
Pediatric-Onset Neuromyelitis Optica Spectrum Disorder: Long-term Follow-up and Therapeutic Challenges in a Treatment-Resistant Case., PMID:40398846
Comparative effectiveness of intravenous versus subcutaneous tocilizumab for refractory uveitis: a retrospective analysis., PMID:40398691
Thyroid eye disease in the biologic era: a 40-year paradigm shift in nonsurgical therapeutic strategies., PMID:40397653
[Clinical phenotype and genotype analysis of neuroinflammation, autoinflammation, splenomegaly and anemia syndrome caused by IRAK4 gene variantion]., PMID:40393760
Combined IVIG and High-Dose Systemic Corticosteroids for the Management of Pembrolizumab-Induced Cytokine Release Syndrome in Lung Adenocarcinoma., PMID:40391237
Tocilizumab Monotherapy or Combined With Methotrexate for Rheumatoid Arthritis: A Randomized Clinical Trial., PMID:40388166
Evolving therapeutic strategies for severe fever with thrombocytopenia syndrome: from past to future., PMID:40385974
Bilateral Alternate Orbital and Ocular Manifestations in a VEXAS Syndrome Patient., PMID:40384388