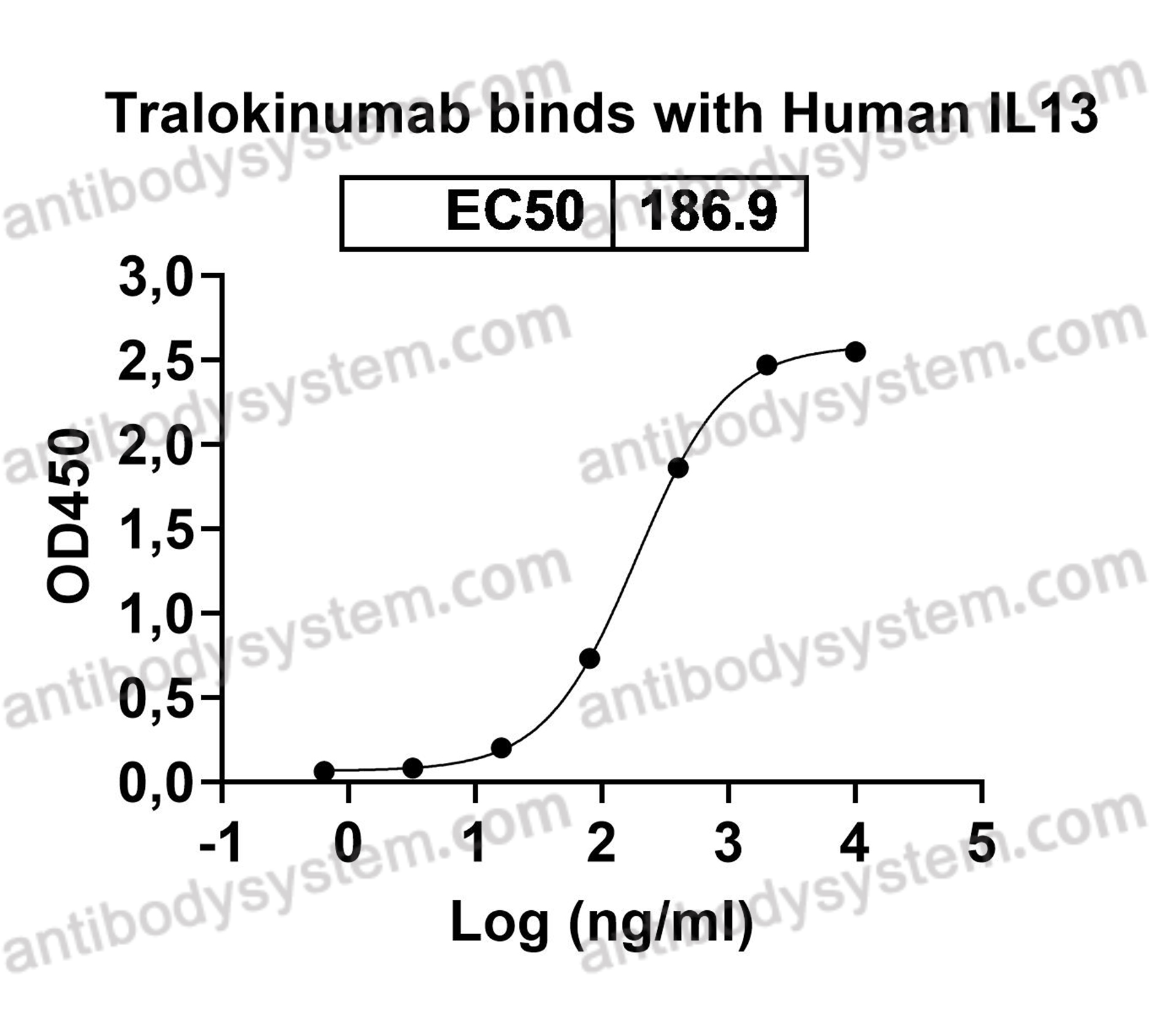
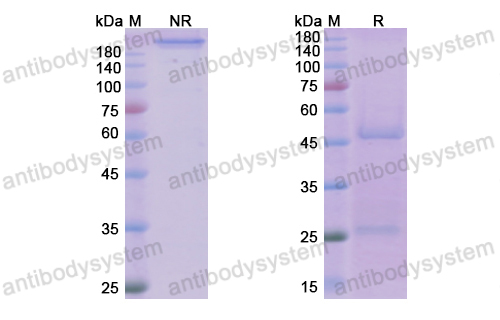
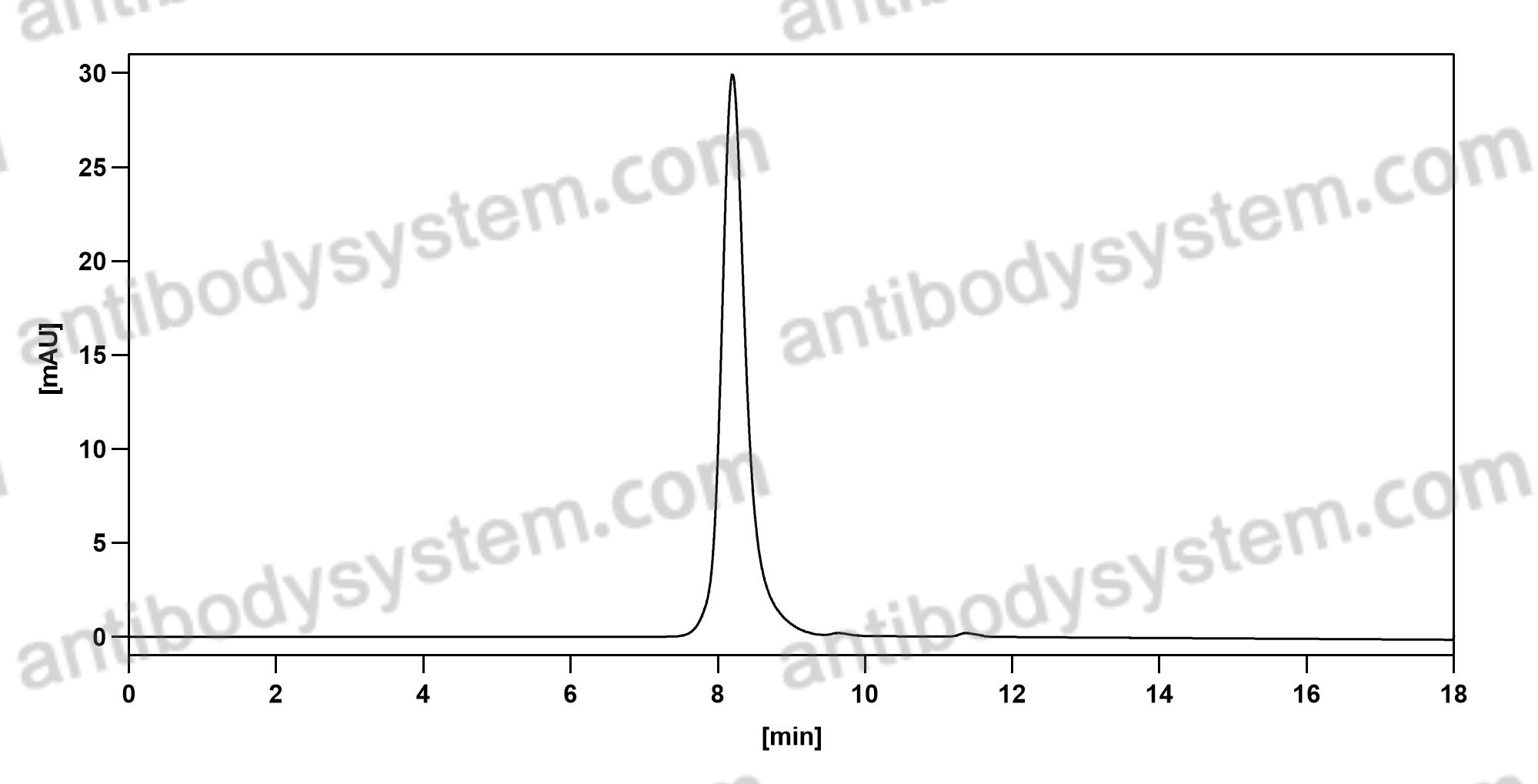
A Phase 2 Randomized Controlled Study of Tralokinumab in Subjects with Idiopathic Pulmonary Fibrosis, PMID: 28787186
A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma, PMID: 22743678
A randomized, placebo-controlled, single ascending-dose study to assess the safety, tolerability, pharmacokinetics, and immunogenicity of subcutaneous tralokinumab in Japanese healthy volunteers, PMID: 29622380
Application of structured statistical analyses to identify a biomarker predictive of enhanced tralokinumab efficacy in phase III clinical trials for severe, uncontrolled asthma, PMID: 31315668
Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis, PMID: 29098604
Biological therapies for atopic dermatitis: An update, PMID: 30679974
Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling, PMID: 32625205
Biologics for Atopic Dermatitis, PMID: 33012322
Commonality of the IL-4/IL-13 pathway in atopic diseases, PMID: 28277826
Current and emerging biologics for the treatment of pediatric atopic dermatitis, PMID: 33078990
Docking analysis and the possibility of prediction efficacy for an anti-IL-13 biopharmaceutical treatment with tralokinumab and lebrikizumab for bronchial asthma, PMID: 29155876
Dose-Exposure-Response Relationship of the Investigational Anti-Interleukin-13 Monoclonal Antibody Tralokinumab in Patients With Severe, Uncontrolled Asthma, PMID: 28758192
Effect of monoclonal antibody drug therapy on mucosal biomarkers in airway disease: A systematic review, PMID: 32808380
Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial, PMID: 29793857
Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial, PMID: 26231288
Efficacy of biologics in atopic dermatitis, PMID: 32003247
Emerging Treatment Options in Atopic Dermatitis: Systemic Therapies, PMID: 29320765
Evaluation of Antibody Properties and Clinically Relevant Immunogenicity, Anaphylaxis, and Hypersensitivity Reactions in Two Phase III Trials of Tralokinumab in Severe, Uncontrolled Asthma, PMID: 30649752
Health-related quality of life with tralokinumab in moderate-to-severe atopic dermatitis: A phase 2b randomized study, PMID: 33333295
Innovation in Atopic Dermatitis: From Pathogenesis to Treatment, PMID: 31964499
Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis, PMID: 31395084
New and Emerging Systemic Treatments for Atopic Dermatitis, PMID: 32519223
New and Emerging Therapies for Pediatric Atopic Dermatitis, PMID: 31364023
New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait!, PMID: 28583618
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis, PMID: 32525769
Pharmacokinetics of tralokinumab in adolescents with asthma: implications for future dosing, PMID: 26182954
Probiotics for treating eczema, PMID: 30480774
Relative efficacy of systemic treatments for atopic dermatitis, PMID: 30296535
Structural Characterisation Reveals Mechanism of IL-13-Neutralising Monoclonal Antibody Tralokinumab as Inhibition of Binding to IL-13Rα1 and IL-13Rα2, PMID: 27956146
The Changing Landscape of Alopecia Areata: The Therapeutic Paradigm, PMID: 28646393
The IL-13-OVOL1-FLG axis in atopic dermatitis, PMID: 31509236
The Safety and Efficacy of Anti-IL-13 Treatment with Tralokinumab (CAT-354) in Moderate to Severe Asthma: A Systematic Review and Meta-Analysis, PMID: 31152798
The tralokinumab story: Nothing is ever simple, PMID: 30659852
Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma, PMID: 30442714
Tralokinumab does not impact vaccine-induced immune responses: Results from a 30-week, randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis, PMID: 33744356
Tralokinumab for atopic dermatitis: a promising new therapy, PMID: 33347600
Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2), PMID: 33000465
Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study, PMID: 25304132
Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials, PMID: 29792288
Tralokinumab for the Treatment of Atopic Dermatitis, PMID: 34155602
Tralokinumab for the treatment of severe, uncontrolled asthma: the ATMOSPHERE clinical development program, PMID: 29536781
Tralokinumab for uncontrolled asthma, PMID: 23268592
Tralokinumab in atopic dermatitis, PMID: 34390128
Tralokinumab pharmacokinetics and tolerability when administered by different subcutaneous injection methods and rates, PMID: 28590244
Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial, PMID: 33000503
Tralokinumab unsuccessful for management of severe, uncontrolled asthma, PMID: 29793858
Tralokinumab: First Approval, PMID: 34406631
Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb, PMID: 29906525
Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches, PMID: 30825336
What's New in Atopic Dermatitis, PMID: 30850043
Long-Term Use of Tralokinumab in Patients With Atopic Dermatitis: A Real-World Multicentric Case Series., PMID:40528669
Corneal Perforation Associated With Tralokinumab., PMID:40525846
Letter: Effectiveness and Safety of Tralokinumab for the Elderly with Atopic Dermatitis in a Real-World Setting-IL AD., PMID:40470535
Real‑World Experience of 1 Year of Tralokinumab Treatment in Adult Patients with Atopic Dermatitis: A Single-Center Retrospective Study., PMID:40459712
Real-World Effectiveness and Safety of Dupilumab, Tralokinumab, and Upadacitinib in Patients with Atopic Dermatitis: A 52-Week International, Multicenter Retrospective Cohort Study., PMID:40459711
Suppression of Il5 and Il13 Gene Expression by Synthetic siRNA Molecules Reduces Nasal Hyperreactivity and Inflammation in a Murine Model of Allergic Rhinitis., PMID:40451198
Real-World Clinical Experience of Tralokinumab in a Tertiary Centre: An Alternative for Patients with Conjunctivitis on Dupilumab?, PMID:40442566
Deciding Which Patients With Atopic Dermatitis to Prioritize for Biologics and Janus Kinase Inhibitors., PMID:40368249
Editor's Highlights-June 2025., PMID:40322884
European Guideline (EuroGuiDerm) on atopic eczema: Living update., PMID:40317496
A Case of Cutaneous Fungal Infection Following the Administration of Dupilumab., PMID:40291282
Correction to: Real-World Evidence of Tralokinumab Effectiveness and Safety: A Systematic Review and Meta-analysis., PMID:40272773
Drug survival of dupilumab, tralokinumab and upadacitinib in patients with atopic dermatitis., PMID:40264378
Real-World pharmacovigilance analysis of drug-related conjunctivitis using the FDA adverse event reporting system database., PMID:40251175
Incidence of upper respiratory tract infections with biological therapies in moderate to severe atopic dermatitis: a systematic review and meta-analysis., PMID:40241901
Long-Term Efficacy and Potential Predictors of Tralokinumab Dose Optimization in Elderly Patients: A Multicentre Study., PMID:40232593
[Chronic Pruritus: A Review of Its Pathophysiology and Current Treatments]., PMID:40214004
Infection risk in atopic dermatitis patients treated with biologics and JAK inhibitors: BioDay results., PMID:40176741
Effectiveness of Switching From Upadacitinib to Tralokinumab in Patients With Moderate-to-Severe Atopic Dermatitis: A Real-World Clinical Practice., PMID:40165566
A Case of Unexpected Successful Treatment of Alopecia Areata With Tralokinumab in a Patient With Atopic Dermatitis., PMID:40152261
Effectiveness of Tralokinumab Across Atopic Dermatitis Phenotypes., PMID:40142885
Real-world experience of switching from tralokinumab to lebrikizumab in atopic dermatitis., PMID:40130939
Long-Term Disease Control and Minimal Disease Activity of Head and Neck Atopic Dermatitis in Patients Treated with Tralokinumab up to 4 Years., PMID:40085349
Investigating Efficacy of Atopic Dermatitis Systemic Therapeutics After Discontinuation Part I: Biologics., PMID:40078859
Effectiveness and Safety of Tralokinumab in Atopic Dermatitis: 1-year Results From a Real-world Multicentre Study., PMID:40077977
Long-term Outcomes of New Systemic Agents in Atopic Dermatitis: Drug Survival Analyses and Treatment Patterns in Daily Practice., PMID:40059465
Real-World Evidence of Tralokinumab Effectiveness and Safety: A Systematic Review and Meta-analysis., PMID:40045152
Comparative Real-World Analysis of Baseline Demographic Characteristics and Comorbidities in Atopic Dermatitis Patients Initiating Biologics Versus JAK Inhibitors., PMID:40004820
Comparative efficacy and safety of dupilumab versus newly approved biologics and JAKi in pediatric atopic dermatitis: A systematic review and network meta-analysis., PMID:39992967
Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2024., PMID:39986987
Lebrikizumab vs Other Systemic Monotherapies for Moderate-to-Severe Atopic Dermatitis: Network Meta-analysis of Efficacy., PMID:39953372
Comparison chart: Interleukin (IL) receptor antagonists for atopic dermatitis., PMID:39946701
Nemolizumab (Nemluvio) for atopic dermatitis., PMID:39946696
Higher Baseline Lactate Dehydrogenase and History of Allergic Rhinitis as Predictive Factors of Conjunctivitis in Atopic Dermatitis Patients Treated with Tralokinumab., PMID:39937140
Effectiveness of Tralokinumab for Different Anatomical Sites and Clinical Signs in Atopic Dermatitis: A 36-Week Real-World Study., PMID:39932721
Drug survival of dupilumab, tralokinumab and upadacitinib in patients with atopic dermatitis: An international, real-world comparative study., PMID:39912554
Erythrodermic atopic dermatitis responding to tralokinumab after dupilumab failure., PMID:39912174
Predictive Factors for Poor Responders to Tralokinumab in Moderate-to-Severe Atopic Dermatitis: A Real-World Analysis., PMID:39907080
Effectiveness and Safety of Dupilumab and Tralokinumab for Treating Atopic Dermatitis and Pruritic Skin Disorders in Oncological Patients: A Narrative Review., PMID:39901963
Diagnosis of cutaneous T-cell lymphoma following exposure to biologic agents for atopic dermatitis: A retrospective cohort study from a single tertiary cancer center., PMID:39900188
Efficacy and Drug Survival of Tralokinumab in Severe Atopic Dermatitis: An 18-Month Multicenter Experience., PMID:39899459
Off-label use of tralokinumab in the treatment of bullous pemphigoid: a case series., PMID:39878153
Real-world experience of tralokinumab for atopic dermatitis: A 16-week multicenter retrospective study., PMID:39864749
Effectiveness of Tralokinumab in Different Phenotypes of Atopic Dermatitis: A Real-World Study., PMID:39862316
Upadacitinib and Dupilumab Demonstrate Superior Efficacy in the Treatment of Adolescent Atopic Dermatitis: A Network Meta-Analysis., PMID:39827864
Correction: Matching-Adjusted Indirect Comparison of the Efficacy at Week 32 of Tralokinumab and Dupilumab in the Treatment of Moderate-to-Severe Atopic Dermatitis., PMID:39821857
Biomarkers in Atopic Dermatitis: A Review of the Role of IL-13 and the Impact of Tralokinumab Treatment., PMID:39820896
Tralokinumab Treatment of Atopic Dermatitis Induces a Progressive Transcriptomic Response., PMID:39733934
Identification of Early and Late Responders to Anti-IL-13 Antibody Tralokinumab in Atopic Dermatitis: A Real-World Japanese Study., PMID:39729342
English version of clinical practice guidelines for the management of atopic dermatitis 2024., PMID:39707640