Catalog No.
KAJ70102
Description
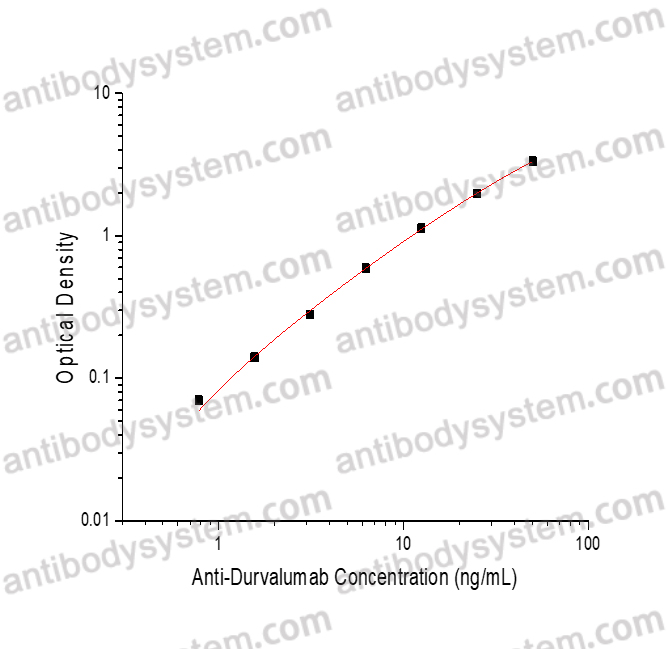
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Durvalumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Durvalumab will be captured by immobilized Durvalumab. After washing away any unbound substances, a biotin-labeled Durvalumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Durvalumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Durvalumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
0.78 - 50 ng/mL
Sensitivity
0.15 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
22.5
|
5.6
|
1.3
|
21.9
|
5.8
|
1.9
|
|
Standard deviation
|
1.7
|
0.3
|
0.1
|
2.4
|
0.3
|
0.3
|
|
CV (%)
|
7.5
|
4.6
|
5.9
|
11.1
|
6.0
|
13.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MEDI4736, CAS: 1428935-60-7
Neutrophil-Lymphocyte Ratio Predicts Overall Survival in Patients With HCC Treated With Durvalumab Plus Tremelimumab., PMID:40515751
First-in-human phase I/II, open-label study of mRNA-2416 alone or combined with durvalumab in patients with advanced solid tumors and ovarian cancer., PMID:40515479
A Critical Review of Immunomodulation in the Management of Inoperable Stage III NSCLC., PMID:40507315
Erythrocytosis as an indicator of disease progression of small cell lung cancer: A case report., PMID:40502212
Ultrasensitive detection and tracking of circulating tumor DNA to predict relapse and survival in patients with locally advanced cervical cancer: phase III CALLA trial analyses., PMID:40500687
Conversion surgery following severe cytokine release syndrome induced by immune checkpoint inhibitors doublet in advanced hepatocellular carcinoma., PMID:40500480
Association of KRAS variants with survival and therapeutic outcomes in biliary tract cancers., PMID:40499463
Central Airway Invasion of Lung Squamous Cell Carcinoma Causing Bronchomediastinal Fistula and Mediastinitis., PMID:40491839
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
Six-year survival after oral temozolomide maintenance therapy in limited-stage small cell lung cancer: A case report., PMID:40487755
Editorial Comment on "Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition - Analysis from a Phase 1b Trial (Durvalumab +/- Tremelimumab)"., PMID:40484289
Durvalumab after concurrent chemoradiotherapy for sensitizing epidermal growth factor receptor-mutant stage III non-small cell lung cancer: A Japanese Real-World data analysis., PMID:40480013
Clinical features, treatment, and outcomes of anti-PD-L1 induced psoriasis., PMID:40474015
Association of candidate surrogate endpoints with overall survival in advanced biliary tract cancer., PMID:40473034
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer., PMID:40454643
A Multimodality Treatment Strategy for an Intrahepatic Cholangiocarcinoma and Complex Liver Resection with Vascular Reconstruction-A Video Vignette., PMID:40450171
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Differential safety profiles of durvalumab monotherapy and durvalumab in combination with tremelimumab in adult patients with advanced cancers., PMID:40447320
Author response to comment on "Phase 2 study of neoadjuvant durvalumab plus docetaxel, oxaliplatin, and S-1 with surgery and adjuvant durvalumab plus S-1 for resectable locally advanced gastric cancer"., PMID:40447315
Treatment of small cell lung cancer; advances and future prospects., PMID:40446464
Safety of Partial and Radical Nephrectomy for Complex Locally Advanced Renal Cell Carcinoma After Neo-Adjuvant Immune Checkpoint Inhibition: Analysis From a Phase 1b Trial (Durvalumab +/- Tremelimumab)., PMID:40441307
Durvalumab after sequential chemoradiotherapy in unresectable stage III non-small-cell lung cancer-final analysis from the phase II PACIFIC-6 trial., PMID:40435873
A novel approach to evaluate the therapeutic efficacy of durvalumab and tremelimumab combination therapy in hepatocellular carcinoma., PMID:40433908
Diagnostic and Therapeutic Challenges in an Older Patient With Concurrent Small-Cell Lung Carcinoma and Primary Duodenal Adenocarcinoma: A Case Report., PMID:40421353
Outcomes in Patients With Resectable Stage III NSCLC Who Did Not Have Definitive Surgery After Neoadjuvant Treatment-A Retrospective Analysis of the SAKK Trials 16/96, 16/00, 16/01, 16/08, and 16/14: A Brief Report., PMID:40420867
[Complete Response Achieved by Gemcitabine+Cisplatin+Durvalumab Therapy for Lymph Node Recurrence of Intraductal Cholangiocarcinoma-A Case Report]., PMID:40420374
Looking Toward the Future: Emerging Therapies for Hepatocellular Carcinoma., PMID:40416920
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
Adjuvant Immunotherapy in Microsatellite Instability-High Colon Cancer: A Literature Review on Efficacy, Challenges, and Future Directions., PMID:40415356
Multicenter phase Ib/II study of second-line durvalumab and tremelimumab in combination with paclitaxel in patients with biomarker-selected metastatic gastric cancer., PMID:40399487
Laparoscopic Extended Segmentectomy 8 with Right Hepatic Vein Resection After Conversion Therapy for Advanced Intrahepatic Cholangiocarcinoma., PMID:40397343
Real-world survival outcomes, treatment patterns, and impact of PD-L1 expression among patients with unresectable, stage III NSCLC treated with CRT → durvalumab in Canada: The RELEVANCE study., PMID:40393235
Factors associated with reaching maintenance therapy in patients with advanced biliary tract cancer treated with durvalumab: Real-world results from a multicenter and multinational study., PMID:40387725
Factors predicting early recurrence in patients with unresectable stage III non-small cell lung cancer on durvalumab consolidation after chemoradiotherapy., PMID:40386716
A platform for SpyCatcher conjugation to native antibodies., PMID:40386161
Immune-mediated adverse events and overall survival with tremelimumab plus durvalumab and durvalumab monotherapy in unresectable hepatocellular carcinoma: HIMALAYA phase 3 randomized clinical trial., PMID:40384092
Durvalumab plus chemotherapy in advanced biliary tract cancer: 3-year overall survival update from the phase III TOPAZ-1 study., PMID:40381735
First-line durvalumab therapy alone or in combination with tremelimumab for metastatic head and neck squamous cell carcinoma: A cost-effectiveness analysis., PMID:40378108
Common Medical Comorbidities Influence Pneumonitis Risk After Chemoradiotherapy and Durvalumab Maintenance in Stage III Non-small Cell Lung Cancer., PMID:40374425
Implementing adjuvant immunotherapy following radical chemoradiotherapy for stage III non-small-cell lung cancer in UK clinical practice-Are the PACIFIC trial outcomes achievable in the real world?, PMID:40371096
Association of Sinonasal Symptoms and Disease With Immune Checkpoint Inhibitor Therapy., PMID:40370338
Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR)., PMID:40361370
A pharmacovigilance analysis of post-marketing safety of durvalumab., PMID:40360595
Equalizing prognostic disparities in KRAS-mutated stage III NSCLC patients: addition of durvalumab to combined chemoradiotherapy improves survival., PMID:40349418
Impact of durvalumab re-administration after moderate symptomatic pneumonitis in locally advanced non-small cell lung cancer., PMID:40349417
Real-world outcomes of subsequent treatment strategies after durvalumab consolidation in stage III unresectable non-small cell lung cancer., PMID:40347676
Carboplatin, etoposide, atezolizumab, and bevacizumab in the first-line treatment of patients with extensive stage small-cell lung cancer: the GOIRC-01-2019 CeLEBrATE study., PMID:40341031
Immune checkpoint inhibitor-associated risk for venous thromboembolism: a comprehensive analysis., PMID:40341027