Catalog No.
KAB86902
Description
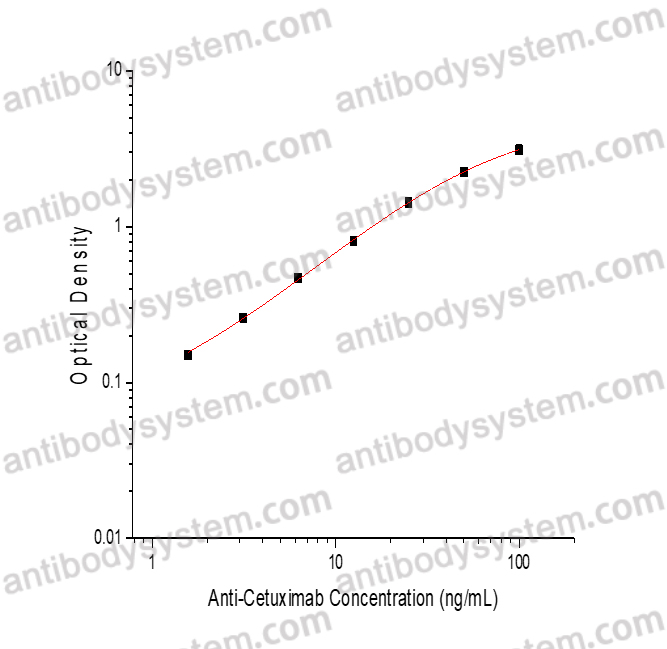
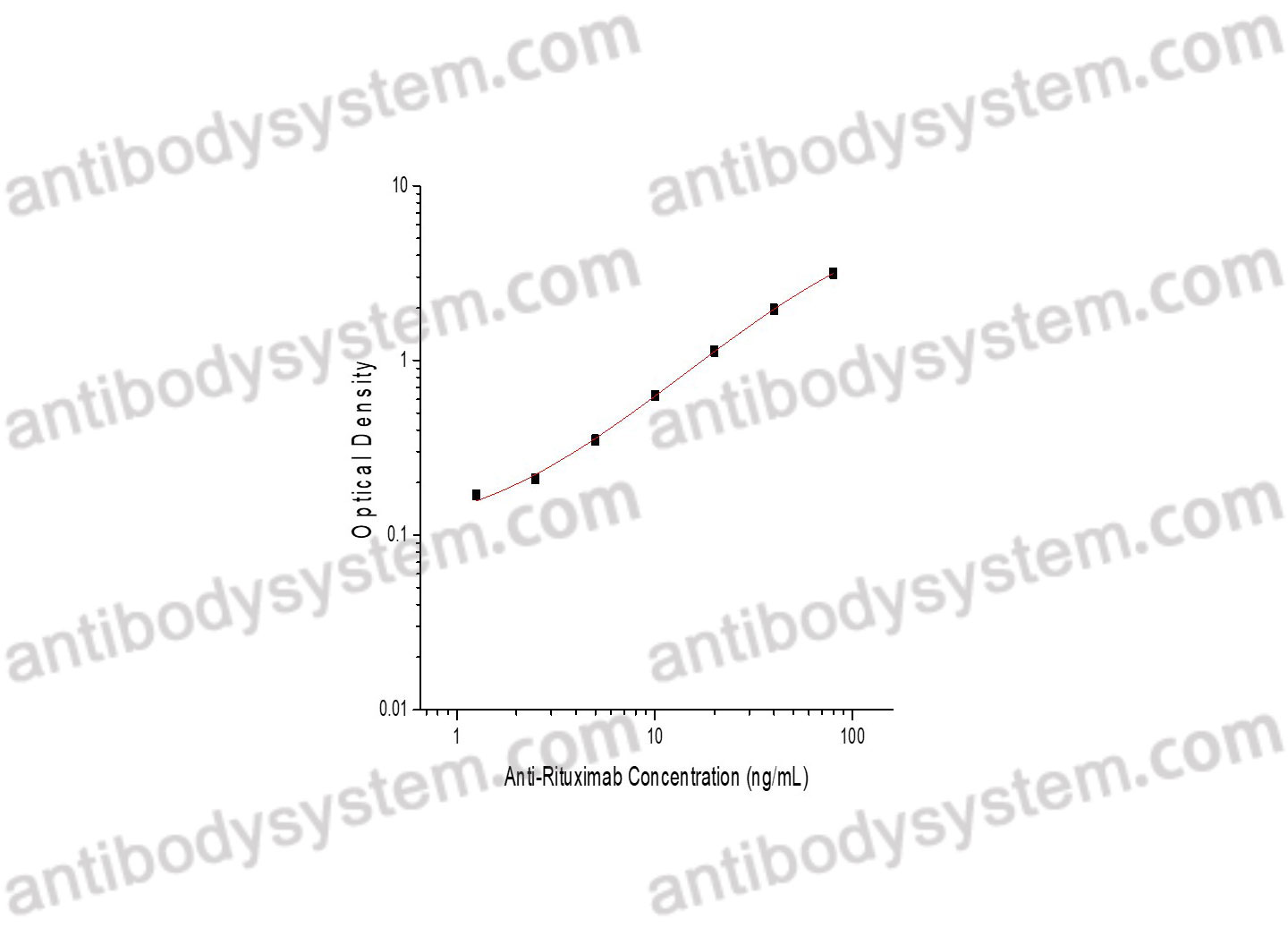
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Cetuximab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Cetuximab will be captured by immobilized Cetuximab. After washing away any unbound substances, a biotin-labeled Cetuximab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Cetuximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Cetuximab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.89 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
46.6
|
12.3
|
2.8
|
46.7
|
12.1
|
3.3
|
|
Standard deviation
|
2.1
|
0.3
|
0.2
|
2.6
|
0.4
|
0.2
|
|
CV (%)
|
4.4
|
2.3
|
7.1
|
5.6
|
3.5
|
6.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
Fab C225,IMC-225,cetuximab-IR700, CAS: 205923-56-4
The Immunology of Alpha-Gal Syndrome: History, Tick Bites, IgE, and Delayed Anaphylaxis to Mammalian Meat., PMID:40515672
LiGeR-HN phase III trials of petosemtamab + pembrolizumab and petosemtamab monotherapy in recurrent or metastatic HNSCC., PMID:40511820
The Prognostic Value of Tumor Fibrosis in Patients Undergoing Hepatic Metastasectomy for Colorectal Cancer: A Retrospective Pooled Analysis., PMID:40507350
Identification and risk modelling of bacterial lipopolysaccharide-related subtypes in head and neck squamous cell carcinoma to predict prognostic and immunological properties., PMID:40505775
Anti-HIV-1 HSPC-based gene therapy with safety kill switch to defend against and attack HIV-1 infection., PMID:40503012
Imbalanced NK cell subpopulations and TIGIT expression limit cetuximab efficacy in colorectal cancer: A promising target for treatment enhancement., PMID:40490938
Epidermal Growth Factor Receptor (EGFR) Downregulation by Cetuximab in Salivary Duct Carcinoma: A Case Report., PMID:40486390
Neoadjuvant therapy-induced immune dynamics and myeloid-associated resistance in advanced head and neck cancer., PMID:40483270
Assessing individual head and neck squamous cell carcinoma patient response to therapy through integration of functional and genomic data., PMID:40473660
Long term feeding tube use in head and neck cancer survivors - a secondary analysis of patient and treatment related factors., PMID:40473230
Retrospective stratified analysis of resistance mechanisms to anti-EGFR therapy in mCRC using tumor tissue samples., PMID:40467730
First-line treatment with a combination of immunotherapy, anti-EGFR monoclonal antibodies, and chemotherapeutics for unresectable left KRAS/BRAF wild-type microsatellite-stable colorectal cancer: a case report., PMID:40460045
Cutaneous adverse effects of combination epidermal growth factor receptor inhibitor and immune checkpoint inhibitor cancer therapy., PMID:40455297
A trispecific antibody targeting EGFR/cMET/VEGF-A demonstrates multiple mechanisms of action to inhibit wild-type and mutant NSCLC animal models., PMID:40452841
Combined inhibition of EGFR and FGFRs with Cetuximab and Infigratinib showed effectiveness and relevance in proliferation and migration of HNSCC cell lines., PMID:40449758
Phase II (Alliance A091802) Randomized Trial of Avelumab Plus Cetuximab Versus Avelumab Alone in Advanced Cutaneous Squamous Cell Carcinoma., PMID:40448574
Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer., PMID:40444708
[Research progress on the mechanisms of resistance to cetuximab targeted therapy in head and neck squamous cell carcinoma]., PMID:40443385
Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review., PMID:40426729
Evaluation of real-time pharmacokinetics using fluorescence imaging system in Near-infrared photoimmunotherapy for head and neck cancer., PMID:40424829
Mitochondrial damage and reactive oxygen species production in C. elegans: key factors in CdTe/ZnS quantum dot-Cet probes., PMID:40421499
Cost-effectiveness of pembrolizumab for the first-line treatment of recurrent or metastatic head and neck squamous cell carcinoma in Colombia., PMID:40417835
Real world treatment patterns for recurrent and metastatic head and neck cancer in the post-KEYNOTE 048 era., PMID:40416868
Basaloid Squamous Cell Carcinoma of the Dorsum of the Tongue Following Chronic Hypertrophic Candidiasis: A Case Report and Literature Review., PMID:40416125
miR-196b strictly regulates and reliably predicts the response to cetuximab in colorectal cancer., PMID:40414859
Exploring Real-World Outcomes of First Line EGFR Inhibitor Use, Cetuximab Versus Panitumumab, in Patients with Left-Sided, RAS Wild-Type, Metastatic Colorectal Cancer., PMID:40413126
The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review., PMID:40407689
Targeted therapy acts to sensitize stereotactic body radiotherapy for pulmonary oligometastases from colorectal cancer., PMID:40406266
Cetuximab plus 5-fluorouracil in patients with advanced cutaneous squamous cell carcinoma: a retrospective cohort study., PMID:40405694
Antibody Labeling With FITC Facilitates Controlled Release From Peptide Hydrogels Bearing Fc-Binding Motifs., PMID:40400208
Application and Perspectives of Immunotherapy in Head and Neck Squamous Cell Carcinoma., PMID:40396808
The potential benefit of encorafenib plus cetuximab with chemotherapy compared with standard of care in people with BRAF V600E-mutant metastatic colorectal cancer in the BREAKWATER study: a plain language summary., PMID:40391894
A platform for SpyCatcher conjugation to native antibodies., PMID:40386161
Cost-effectiveness of cetuximab-containing regimens for squamous cell carcinoma of the head and neck in Italy., PMID:40383966
First-line durvalumab therapy alone or in combination with tremelimumab for metastatic head and neck squamous cell carcinoma: A cost-effectiveness analysis., PMID:40378108
Neoadjuvant Encorafenib Plus Cetuximab in BRAF-V600E-Mutated Locally Advanced Colon Cancer., PMID:40373261
A phase Ib study of photoimmunotherapy with ASP-1929 in combination with nivolumab for advanced gastric cancer (GE-PIT, EPOC1901)., PMID:40372585
Evaluation of the usefulness of protocol-based magnesium supplementation for hypomagnesemia in patients with advanced or recurrent colorectal cancer treated with panitumumab., PMID:40366467
Employing the SpyTag-SpyCatcher Reaction for the Modification of Supramolecular Polymers with Functional Proteins., PMID:40365870
Dual Disruption of EGFR/PI3K Signaling: IGF2BP2 Targeting Reverses Anti-EGFR Resistance in CAFs-Infiltrated Oral Squamous Cell Carcinoma., PMID:40362183
[A Case of Complete Cured by Multidisciplinary Treatment for Repeated Recurrent of Primary Peritoneal Cancer]., PMID:40360415
Ligand-activated EGFR/MAPK signaling but not PI3K, are key resistance mechanisms to EGFR-therapy in colorectal cancer., PMID:40346041
Resveratrol improved atherosclerosis by increasing LDLR levels via the EGFR-ERK1/2 signaling pathway., PMID:40340973
Targeting the HER2-ELF3-KRAS axis: a novel therapeutic strategy for KRASG13D colorectal cancer., PMID:40340861
A plain language summary of the molecular changes in the tumors of people with BRAF V600E-mutant colorectal cancer in the BEACON study., PMID:40340632
PROshot: Radiation Alone for Low-risk, Early-stage Breast Cancer, Stereotactic Body Radiation Therapy for Advanced and Recurrent Hepatocellular Carcinoma, and the Role of Cetuximab in Treating Head and Neck Squamous Cell Carcinoma., PMID:40340074
Genome-wide DNA methylation status as a biomarker for clinical outcomes of first-line treatment in patients with RAS wild-type metastatic colorectal cancer: JACCRO CC-13AR., PMID:40334314
Experimental System Design and Modelling of EGFR Extracellular Domain and Its Mutant Binding to Antibody Interacting Partner., PMID:40332084
Molecular Characterization and Clinical Outcomes of Pancreatic Neuroendocrine Neoplasms Harboring PAK4-NAMPT Alterations., PMID:40330142
Combining Cetuximab and Immunotherapy for Treating MSS/pMMR Colorectal Cancer: Current Evidence and Challenges., PMID:40329596