Catalog No.
KAV18001
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. SARS-CoV-2 Nucleocapsid has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Nucleocapsid (NP) Human IgG present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Nucleocapsid (NP) Human IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Target
Nucleocapsid, NP
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Nucleocapsid (NP) Human IgG concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
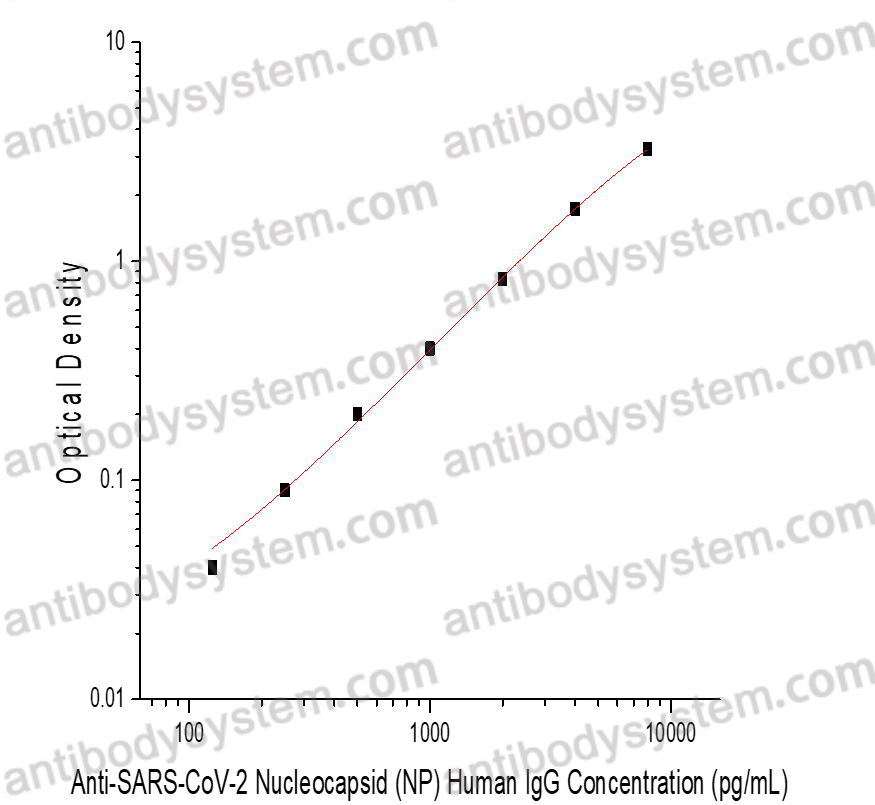
Range
125 - 8,000 pg/mL
Sensitivity
100 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022., PMID:38169905
Characterization of SARS-CoV-2-specific humoral immunity and associated factors in the healthy population post-vaccination., PMID:38103966
SARS-CoV-2 seroprevalence determination in pets and camels in Egypt using multispecies enzyme-linked immunosorbent assay., PMID:38061231
Kinetics of specific anti-SARS-CoV-2 IgM, IgA, and IgG responses during the first 12 months after SARS-CoV-2 infection: A prospective longitudinal study., PMID:37437051
Evaluation of SARS-CoV-2 Specific Antibodies in Recovered Patients by Different ELISA Kits., PMID:37395262
The detection of IgG class antibodies against SARS-CoV-2 nucleocapsid protein by application of nanoparticles., PMID:36808369
Accuracy of serological tests for COVID-19: A systematic review and meta-analysis., PMID:36589993
SARSPLEX: Multiplex Serological ELISA with a Holistic Approach., PMID:36560597
A Novel Dry-Stabilized Whole Blood Microsampling and Protein Extraction Method for Testing of SARS-CoV-2 Antibody Titers., PMID:36298625
SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays., PMID:36146844
DEVELOPMENT AND VALIDATION OF AN ENZYME-LINKED IMMUNOASSAY KIT FOR DIAGNOSIS AND SURVEILLANCE OF COVID-19., PMID:35993012
Development of robust, indigenous ELISA for detection of IgG antibodies against CoV-2 N and S proteins: mass screening., PMID:35976427
Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19., PMID:35959109
Evaluation of Performance of Detection of Immunoglobulin G and Immunoglobulin M Antibody Against Spike Protein of SARS-CoV-2 by a Rapid Kit in a Real-Life Hospital Setting., PMID:35558113
Seroprevalence and immunological memory against SARS-CoV-2 in lung cancer patients: the SOLID study., PMID:35242627
Estimating SARS-CoV-2 Seroprevalence in Canadian Blood Donors, April 2020 to March 2021: Improving Accuracy with Multiple Assays., PMID:35196819
IgG antibody titers against SARS-CoV-2 nucleocapsid protein correlate with the severity of COVID-19 patients., PMID:34922455
Antibody Responses to SARS-CoV-2 Infection-Comparative Determination of Seroprevalence in Two High-Throughput Assays versus a Sensitive Spike Protein ELISA., PMID:34835241
Rapid antibody testing for SARS-CoV-2 vaccine response in pediatric healthcare workers., PMID:34601142
[Herd immunity to SARS-CoV-2 in the Novosibirsk Region population amid the COVID-19 pandemic]., PMID:34545722
Development of a Nucleocapsid Protein-Based ELISA for Detection of Human IgM and IgG Antibodies to SARS-CoV-2., PMID:33869946
Application of newly developed SARS-CoV2 serology test along with real-time PCR for early detection in health care workers and on-time plasma donation., PMID:33869895
Comparative evaluation of SARS-CoV-2 IgG assays against nucleocapsid and spike antigens., PMID:33720878
Automated Western immunoblotting detection of anti-SARS-CoV-2 serum antibodies., PMID:33660134
Development and performance evaluation of a rapid in-house ELISA for retrospective serosurveillance of SARS-CoV-2., PMID:33529223
Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays., PMID:33319585
Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak., PMID:32681308