Catalog No.
KVV18001
Target
Nucleocapsid, NP
Applications
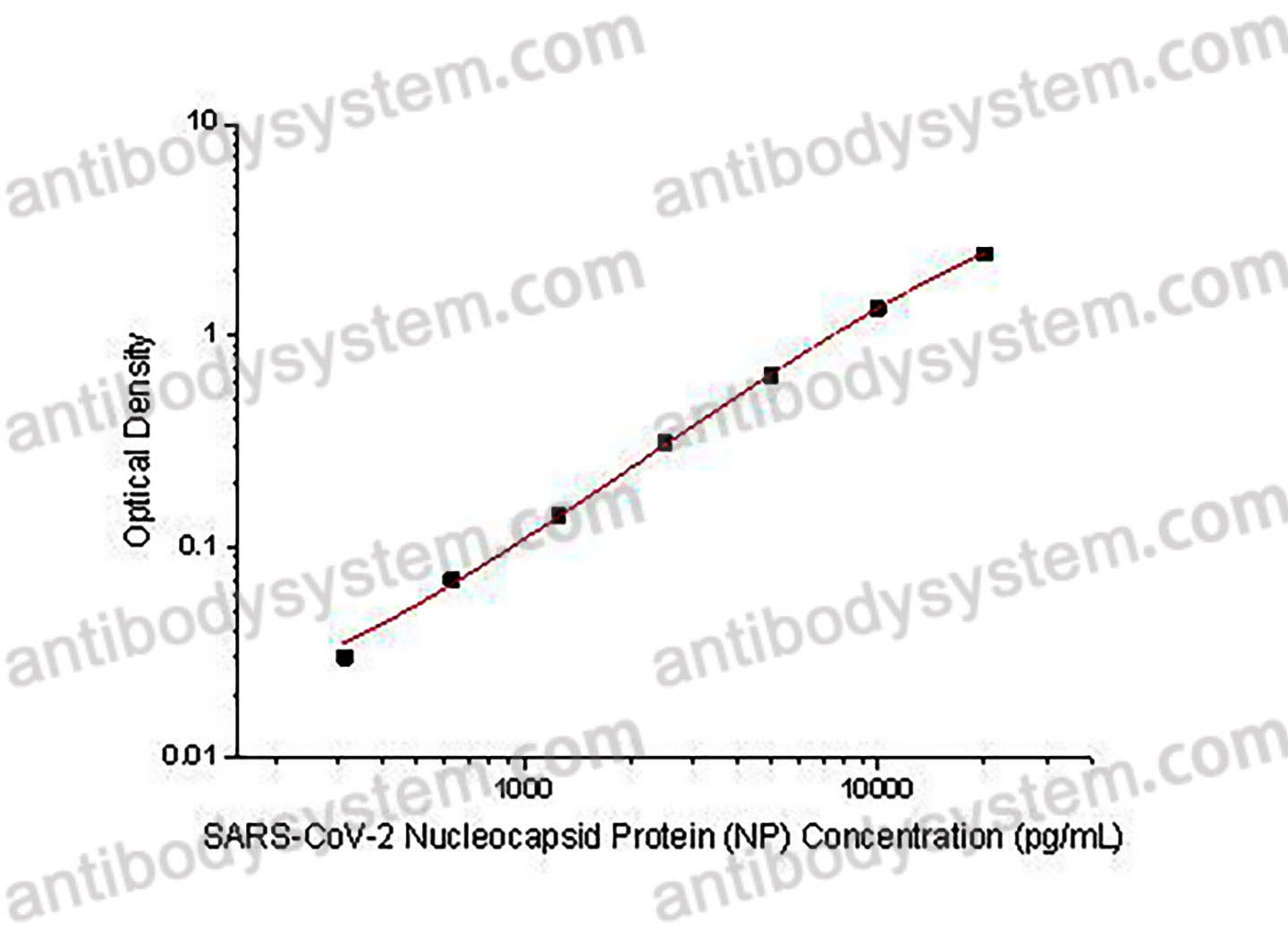
Used for the quantitative determination of SARS-CoV-2 Nucleocapsid Protein (NP) concentration in bronchoalveolar lavage fluid and nasopharyngeal swab samples.
Detection method
Colorimetric
Sample type
Serum, Plasma and Cell culture supernatant.
Assay type
Quantitative
Range
312.5 - 20,000 pg/mL
Sensitivity
167.31 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (pg/mL)
|
9633.1
|
2956.4
|
1027.6
|
10199.5
|
2764.3
|
988.2
|
|
Standard deviation
|
573.1
|
118.3
|
58.3
|
599.1
|
139.3
|
84.6
|
|
CV (%)
|
5.9
|
4.0
|
5.7
|
5.9
|
5.0
|
8.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Dynamic ensembles of SARS-CoV-2 N-protein reveal head-to-head coiled-coil-driven oligomerization and phase separation., PMID:40503686
Single-particle quantification of SARS-CoV-2 virus-like particles using flow virometry., PMID:40483334
Seroprevalence Trends of Antibodies to SARS-CoV-2 in South Korea, 2021-2022: A Repeated Cross-Sectional Study., PMID:40462400
SARS-CoV-2-specific humoral immunity in a Norwegian cohort between 2020 and 2023., PMID:40462062
A Nanomaterial-Independent Biosensor Based on Gallium Arsenide High-Electron-Mobility Transistors for Rapid and Ultra-Sensitive Pathogen Detection., PMID:40461437
SARS-CoV-2 N protein exerts antitumor effects in NSCLC by inducing DNA damage and augmenting chemotherapeutic sensitivity., PMID:40451965
Evidence of SARS-CoV-2 bacteriophage potential in human gut microbiota., PMID:40444030
The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil., PMID:40430765
Screening bacterial effectors and human virus proteins in yeast to identify host factors driving tombusvirus RNA recombination: a role for autophagy and membrane phospholipid content., PMID:40422074
Intrinsic Factors Behind Long COVID: VI. Combined Impact of G3BPs and SARS-CoV-2 Nucleocapsid Protein on the Viral Persistence and Long COVID., PMID:40415285
SERS-based lateral flow immunoassay for rapid and sensitive sensing of nucleocapsid protein toward SARS-CoV-2 screening in clinical samples., PMID:40409906
Phosphorylation Changes SARS-CoV-2 Nucleocapsid Protein's Structural Dynamics and Its Interaction With RNA., PMID:40375582
Natural product sennoside B disrupts liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein by inhibiting its RNA-binding activity., PMID:40371698
Development of monoclonal antibodies against SARS-CoV-2 nucleocapsid protein for COVID-19 antigen detection., PMID:40361217
Transfer of SARS-CoV-2 nucleocapsid protein to uninfected epithelial cells induces antibody-mediated complement deposition., PMID:40343796
SARS-CoV-2 N protein interacts with SLC7A11 to cause ferroptosis in acute lung injury., PMID:40342111
Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with lung infection and tissue-damage biomarkers., PMID:40339608
High-resolution structure reveals enhanced 14-3-3 binding by a mutant SARS-CoV-2 nucleoprotein variant with improved replicative fitness., PMID:40318379
Ultra-sensitive heterojunction double gate BioTFET device for SARS-CoV-2 biomolecules detection., PMID:40307462
Variant mutation G215C in SARS-CoV-2 nucleocapsid enhances viral infection via altered genomic encapsidation., PMID:40299982
Identification and Characterization of a Novel Protein-Protein Interaction Among SARS-CoV-2 Nucleocapsid, Host SFPQ, and hnRNP U and Its Potential Role in Virus Replication., PMID:40259455
SARS-CoV-2 nucleocapsid protein induces a Mincle-dependent macrophage inflammatory response in acute kidney injury., PMID:40244324
Construction and immunogenicity analysis of a recombinant baculovirus targeting the N protein of SARS-CoV-2., PMID:40237823
Optimization of A549 Cell Transfection Efficiency with a Plasmid Encoding the N-Protein of the SARS-CoV-2 Virus., PMID:40216710
Targeting USP22 to promote K63-linked ubiquitination and degradation of SARS-CoV-2 nucleocapsid protein., PMID:40183543
Detection of IgG Antibodies Against COVID-19 N-Protein by Hybrid Graphene-Nanorod Sensor., PMID:40136961
Nucleocapsid-directed antibody testing is unsuitable to estimate hybrid immunity against SARS-CoV-2, a longitudinal cross-border study in the Meuse-Rhine Euroregion., PMID:40132351
Increase of VEGF and Fibronectin expression and ultrastructural alterations of intercellular junctions in a swab negative patient after SARS-COV-2 infection., PMID:40114185
Structural insights into the RNA binding inhibitors of the C-terminal domain of the SARS-CoV-2 nucleocapsid., PMID:40113149
Time course and determinants of the antibody response to SARS-CoV-2 in Costa Rica: the RESPIRA study., PMID:40102764
Chemokines simultaneously bind SARS-CoV-2 nucleocapsid protein RNA-binding and dimerization domains., PMID:40097964
Targeting the liquid-liquid phase separation of nucleocapsid broadly inhibits the replication of SARS-CoV-2 strains., PMID:40086356
Characterization of the binding features between SARS-CoV-2 5'-proximal transcripts of genomic RNA and nucleocapsid proteins., PMID:40077853
Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells., PMID:40076291
Ultrasensitive, Real-Time Detection of Viral Antigens and RNA Enabled by Scalable Graphene-Based FET Sensors for Pathogen Detection: A Case Study on COVID-19., PMID:40073430
Vaccination with Plasmids Encoding the Fusion Proteins D-S1, D-S1N and O-SN from SARS-CoV-2 Induces an Effective Humoral and Cellular Immune Response in Mice., PMID:40006682
Immunoassay Detection of SARS-CoV-2 Using Monoclonal Antibody Binding to Viral Nucleocapsid Protein., PMID:39989430
Development of a Peptide Aptamer-Based TRFIA for the Quantitive Detection of SARS-CoV-2 Nucleocapsid Protein., PMID:39985615
"All-U-Want" Strand Displacement Amplification: A Versatile Signal Amplification Method for Nucleic Acid Biosensing., PMID:39951690
Characterising the SARS-CoV-2 nucleocapsid (N) protein antibody response., PMID:39922387
Revealing the Potential of a Chimaera: a Peptide-Peptide Nucleic Acid Molecule Designed To Interact with the SARS-CoV-2 Nucleocapsid Protein., PMID:39912211
Comparative evaluation of cell-based assay technologies for scoring drug-induced condensation of SARS-CoV-2 nucleocapsid protein., PMID:39894078
Effectiveness of Frequent Point-of-Care Molecular COVID-19 Surveillance in a Rural Workplace: Nonrandomized Controlled Clinical Trial Among Miners., PMID:39869851
An electrochemical microsensor of the SARS-CoV-2 nucleocapsid protein based on a surface-imprinted acupuncture needle., PMID:39865998
Structural insights into nucleocapsid protein variability: Implications for PJ34 efficacy against SARS-CoV-2., PMID:39848104
The R203M and D377Y mutations of the nucleocapsid protein promote SARS-CoV-2 infectivity by impairing RIG-I-mediated antiviral signaling., PMID:39841800
SARS-CoV-2 Is an Electricity-Driven Virus., PMID:39838019
Retraction of: Structural Insights of the SARS-CoV-2 Nucleocapsid Protein: Implications for the Inner-workings of Rapid Antigen Tests., PMID:39837351
The Blood-Cerebrospinal Fluid Barrier as a Potential Entry Site for the SARS-CoV-2 Virus., PMID:39835622
Plasmon-Enhanced Fluorescence Paper Lateral Flow Strip for Point-of-Care Testing of SARS-CoV-2 Antigens., PMID:39834249