Catalog No.
KAV00110
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 Spike Protein (Trimer) has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Spike Protein (Trimer) Human IgA present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgA antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Spike Protein (Trimer) Human IgA bound in the initial step. The color development is stopped and the intensity of the color is measured.
Target
Spike
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Spike Protein (Trimer) Human IgA concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
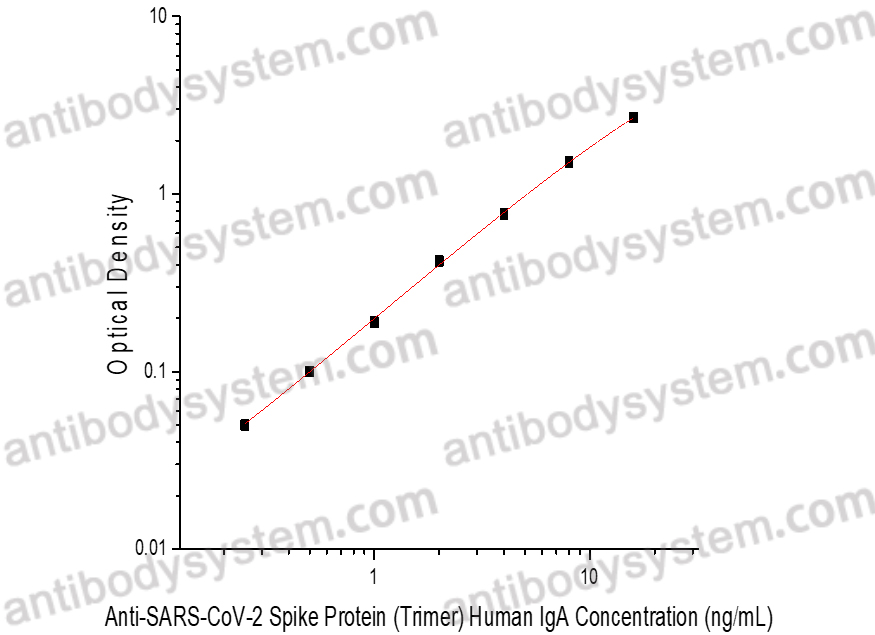
Range
0.25 - 16 ng/mL
Sensitivity
0.07 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
7.59
|
1.82
|
0.42
|
7.57
|
1.82
|
0.41
|
|
Standard deviation
|
0.20
|
0.07
|
0.02
|
0.30
|
0.10
|
0.02
|
|
CV (%)
|
2.60
|
4.08
|
5.05
|
3.91
|
5.57
|
3.94
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Construction of a variable fragment (Fv)-immunoglobulin A (IgA) anti-receptor binding domain (RBD) SARS-CoV-2 library based on IgA from Indonesian COVID-19 survivors., PMID:40403817
The Combination of TLR4 and TLR9 Agonists with Self-Amplifying RNA Lipid Nanoparticles Leads to a More Powerful Immune Response Against SARS-CoV-2., PMID:40401447
Mucosal unadjuvanted booster vaccines elicit local IgA responses by conversion of pre-existing immunity in mice., PMID:40360777
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity., PMID:40359129
Mimicking immune complexes for efficient antibody responses., PMID:40356891
Engineered bacteria as an orally administered anti-viral treatment and immunization system., PMID:40340796
Comprehensive analysis of nasal IgA antibodies induced by intranasal administration of the SARS-CoV-2 spike protein., PMID:40338637
Mucosal immune responses to SARS-CoV-2 infection and COVID-19 vaccination., PMID:40311214
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge., PMID:40258004
Nasal delivery of killed Bacillus subtilis spores protects against influenza, RSV and SARS-CoV-2., PMID:40242757
Exploring TLR agonists as adjuvants for COVID-19 oral vaccines., PMID:40184639
Study protocol for a randomised controlled trial evaluating the efficacy of dietary modulation of probiotics on nutritional status and antibody response to SARS-CoV-2 in Indonesian adolescents: gut-lung axis (DIVINE)., PMID:40180370
Immunogenicity and safety of monovalent and bivalent SARS-CoV-2 variant adapted RBD-based protein booster vaccines in adults previously immunized with different vaccine platforms: A phase II/III, randomized clinical trial., PMID:40179522
Virus-specific antibody responses in severe acute respiratory syndrome coronavirus 2-infected and vaccinated individuals., PMID:40157431
Prior SARS-CoV-2 infection affects adaptive immune responses to Omicron BA.4/BA.5 mRNA booster., PMID:40044048
Association of infection-induced antibody levels with risk of subsequent SARS-COV-2 reinfection among healthcare professionals, Rhode Island, 1 March 2020-17 February 2021., PMID:39998388
Intranasal recombinant protein subunit vaccine targeting TLR3 induces respiratory tract IgA and CD8 T cell responses and protects against respiratory virus infection., PMID:39983329
Validation and clinical performance of a non-commercial ELISA for SARS-CoV-2 anti-RBD IgA antibodies., PMID:39894142
mRNA vaccine-induced SARS-CoV-2 spike-specific IFN-γ and IL-2 T-cell responses are predictive of serological neutralization and are transiently enhanced by pre-existing cross-reactive immunity., PMID:39887249
IgA class switching enhances neutralizing potency against SARS-CoV-2 by increased antibody hinge flexibility., PMID:39828085
Oral Immunisation With Non-GMO Surface Displayed SARS-CoV-2 Spike Epitopes on Bacteria-Like Particles Provokes Robust Humoral and Cellular Immune Responses, and Modulated the Gut Microbiome in Mice., PMID:39797809
Evaluation of anti-SARS-CoV-2 RBD antibody response after booster dose of SpikoGen® in individuals with two previous doses of Sinopharm and its association with HLA-DR and -DQ alleles., PMID:39764935
A SARS-CoV-2 mucosal nanovaccine based on assembly of maltodextrin, STING agonist and polyethyleneimine., PMID:39756748
SARS-CoV-2 immune responses in patients with multiple myeloma and lenalidomide maintenance therapy., PMID:39744626
Optimizing immunogenicity and product presentation of a SARS-CoV-2 subunit vaccine composition: effects of delivery route, heterologous regimens with self-amplifying RNA vaccines, and lyophilization., PMID:39737197
Unraveling the impact of SARS-CoV-2 mutations on immunity: insights from innate immune recognition to antibody and T cell responses., PMID:39720734
Mucosal SARS-CoV-2 S1 adenovirus-based vaccine elicits robust systemic and mucosal immunity and protects against disease in animals., PMID:39629990
Comparable and sustained levels of S1-RBD-IgG and S1-RBD-IgA in BNT162b2 homologous and CoronaVac-BNT162b2 heterologous booster vaccination: A 22-month prospective study in Malaysia., PMID:39490114
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge., PMID:39372768
Unravelling the role of secretory Immnuoglobulin-A in COVID-19: a multicentre study in nursing homes during the first wave., PMID:39354348
An intranasally administered adenovirus-vectored SARS-CoV-2 vaccine induces robust mucosal secretory IgA., PMID:39315545
Reassuring humoral and cellular immune responses to SARS-CoV-2 vaccination in participants with systemic sclerosis., PMID:39305938
The immune response to Covid-19 mRNA vaccination among Lymphoma patients receiving anti-CD20 treatment., PMID:39295862
Monitoring SARS-CoV-2 IgA, IgM and IgG antibodies in dried blood and saliva samples using antibody proximity extension assays (AbPEA)., PMID:39289450
Intranasal HD-Ad-FS vaccine induces systemic and airway mucosal immunities against SARS-CoV-2 and systemic immunity against SARS-CoV-2 variants in mice and hamsters., PMID:39281669
Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant., PMID:39273405
Early, Robust Mucosal Secretory Immunoglobulin A but not Immunoglobulin G Response to Severe Acute Respiratory Syndrome Coronavirus 2 Spike in Oral Fluid Is Associated With Faster Viral Clearance and Coronavirus Disease 2019 Symptom Resolution., PMID:39269503
Sequence Matters: Primary COVID-19 Vaccination after Infection Elicits Similar Anti-spike Antibody Levels, but Stronger Antibody Dependent Cell-mediated Cytotoxicity than Breakthrough Infection., PMID:39248629
Immunogenicity and efficacy of XBB.1.5 rS vaccine against the EG.5.1 variant of SARS-CoV-2 in Syrian hamsters., PMID:39230305
Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates., PMID:39227514
Salivary immune responses after COVID-19 vaccination., PMID:39226256
Intranasal Administration of Recombinant Newcastle Disease Virus Expressing SARS-CoV-2 Spike Protein Protects hACE2 TG Mice against Lethal SARS-CoV-2 Infection., PMID:39204044
Intranasal Self-Adjuvanted Lipopeptide Vaccines Elicit High Antibody Titers and Strong Cellular Responses against SARS-CoV-2., PMID:39196071
The Healthcare Study Examines the Humoral Anti-S1 Antibody Response Following mRNA Vaccination, Comparing Individuals with and without Prior SARS-CoV-2 Infection., PMID:39146978
Persistent and robust antibody responses to ChAdOx1-S Oxford-AstraZeneca (ChAdOx1-S, Covishield) SARS-CoV-2 vaccine observed in Ugandans across varied baseline immune profiles., PMID:39074077
Intranasally Inoculated SARS-CoV-2 Spike Protein Combined with Mucoadhesive Polymer Induces Broad and Long-Lasting Immunity., PMID:39066433
Factors Influencing Breast Milk Antibody Titers during the Coronavirus Disease 2019 Pandemic: An Observational Study., PMID:39064762
Performance assessment of a new serological diagnostic test for COVID-19 with candidate peptides from spike and nucleocapsid viral proteins., PMID:39042245
Intranasal adenovirus-vectored Omicron vaccine induced nasal immunoglobulin A has superior neutralizing potency than serum antibodies., PMID:39039046