Catalog No.
KAV00304
Description
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 RBD has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Human Anti-SARS-CoV-2 RBD IgG present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Human Anti-SARS-CoV-2 RBD IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Target
RBD
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 RBD Human IgG concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
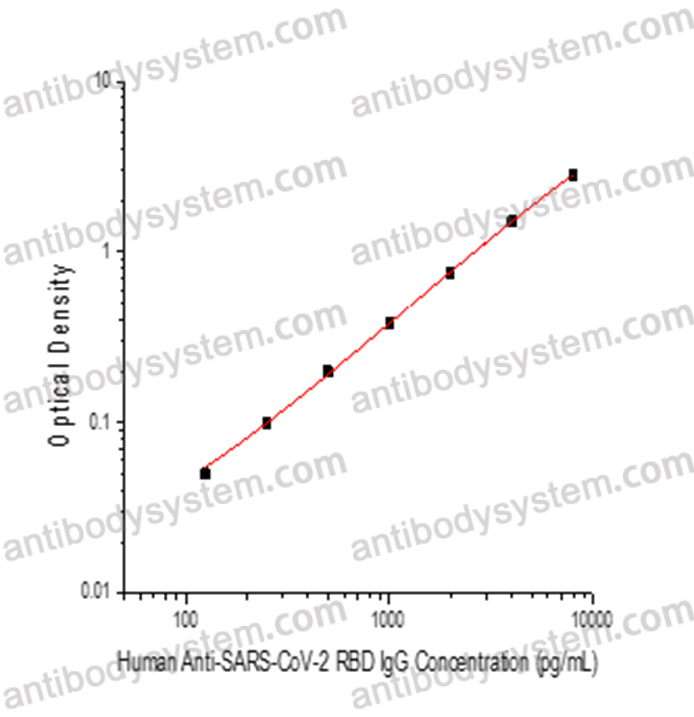
125 - 8,000 pg/mL
Sensitivity
52.31 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (pg/mL)
|
3190.88
|
569.64
|
160.59
|
3521.55
|
669.60
|
166.70
|
|
Standard deviation
|
95.73
|
28.13
|
6.66
|
90.00
|
34.72
|
12.38
|
|
CV (%)
|
3.00
|
4.94
|
4.15
|
2.56
|
5.18
|
7.43
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Longitudinal Follow-Up of the Specific Antibody Response to SARS-CoV-2 Vaccination in Colombia., PMID:39817585
Snapshot of Anti-SARS-CoV-2 IgG Antibodies in COVID-19 Recovered Patients in Guinea., PMID:38792506
Efficient transplacental transfer of SARS-CoV-2 antibodies between naturally exposed mothers and infants in Accra, Ghana., PMID:38730052
Microfluidic particle counter visualizing mucosal antibodies against SARS-CoV-2 in the upper respiratory tract for rapid evaluation of immune protection., PMID:38660972
Characterization of SARS-CoV-2-specific humoral immunity and associated factors in the healthy population post-vaccination., PMID:38103966
Increasing Levels of Serum Anti-Spike S1-RBD IgG after 120 Days of the Pfizer-BioNTech-mRNA Second Dose Vaccination., PMID:38028836
Kinetics of specific anti-SARS-CoV-2 IgM, IgA, and IgG responses during the first 12 months after SARS-CoV-2 infection: A prospective longitudinal study., PMID:37437051
Designing and developing a sensitive and specific SARS-CoV-2 RBD IgG detection kit for identifying positive human samples., PMID:36871661
Development and Evaluation of a Rapid Neutralizing Antibody Assay for COVID-19 Vaccination., PMID:36278077
A Versatile Biomimic Nanotemplating Fluidic Assay for Multiplex Quantitative Monitoring of Viral Respiratory Infections and Immune Responses in Saliva and Blood., PMID:36253095
SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays., PMID:36146844
Safety and immunogenicity of a recombinant receptor-binding domain-based protein subunit vaccine (Noora vaccine™) against COVID-19 in adults: A randomized, double-blind, placebo-controlled, Phase 1 trial., PMID:36029105
Development of robust, indigenous ELISA for detection of IgG antibodies against CoV-2 N and S proteins: mass screening., PMID:35976427
Efficacy of Sinopharm® COVID-19 Vaccine in Hemodialysis Patients: A Preliminary Report., PMID:35962641
Evaluation of Performance of Detection of Immunoglobulin G and Immunoglobulin M Antibody Against Spike Protein of SARS-CoV-2 by a Rapid Kit in a Real-Life Hospital Setting., PMID:35558113
Anti-SARS-CoV-2 IgG and Neutralizing Antibody Levels in Patients with Past COVID-19 Infection: A Longitudinal Study., PMID:35378574
Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant., PMID:35198159
Estimating SARS-CoV-2 Seroprevalence in Canadian Blood Donors, April 2020 to March 2021: Improving Accuracy with Multiple Assays., PMID:35196819
Timeline of SARS-CoV-2 Spread in Italy: Results from an Independent Serological Retesting., PMID:35062265
Development and validation of novel kit for quantification of SARS-CoV-2 antibodies on clinical samples., PMID:34919976
Two novel SARS-CoV-2 surrogate virus neutralization assays are suitable for assessing successful immunization with mRNA-1273., PMID:34563583
Evaluation of a Commercial Culture-Free Neutralization Antibody Detection Kit for Severe Acute Respiratory Syndrome-Related Coronavirus-2 and Comparison With an Antireceptor-Binding Domain Enzyme-Linked Immunosorbent Assay., PMID:34136587
Robust and low-cost ELISA based on IgG-Fc tagged recombinant proteins to screen for anti-SARS-CoV-2 antibodies., PMID:34051226
COVID-19 in Russia: Clinical and Immunological Features of the First-Wave Patients., PMID:33959390
Application of newly developed SARS-CoV2 serology test along with real-time PCR for early detection in health care workers and on-time plasma donation., PMID:33869895