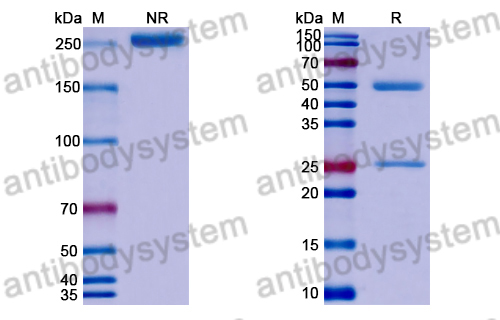
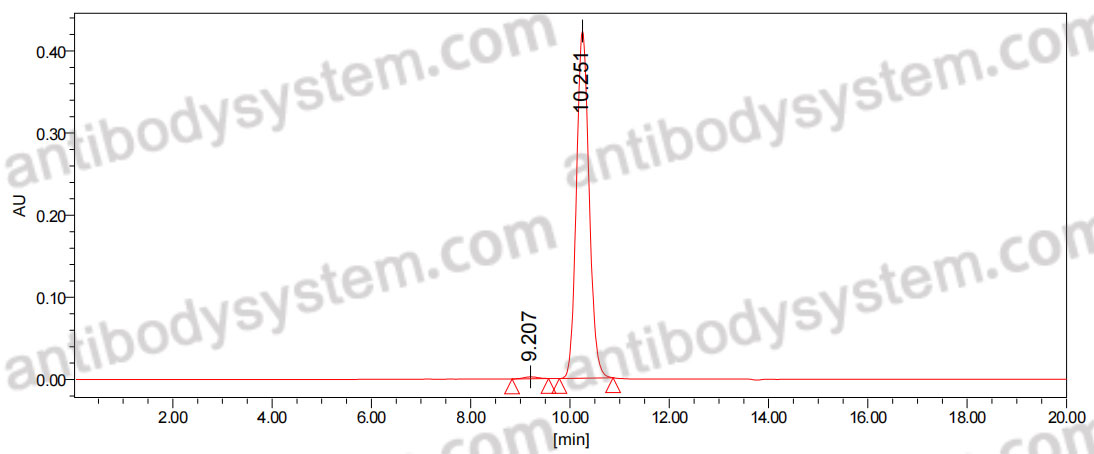
IL-33 protects from recurrent C. difficile infection by restoration of humoral immunity., PMID:40048372
Safety and Immunogenicity of an Adjuvanted Clostridioides difficile Vaccine Candidate in Healthy Adults: A Randomized Placebo-Controlled Phase 1 Study., PMID:39447053
Development of two recombinant vaccines against Clostridioides difficile infection and immunogenicity in pregnant sows and neonatal piglets., PMID:39127403
Fit-for-purpose heterodivalent single-domain antibody for gastrointestinal targeting of toxin B from Clostridium difficile., PMID:38923049
A sequence invariable region in TcdB2 is required for toxin escape from Clostridioides difficile., PMID:38888328
Clostridioides difficile toxin B subverts germinal center and antibody recall responses by stimulating a drug-treatable CXCR4-dependent mechanism., PMID:38761377
The impact of existing total anti-toxin B IgG immunity in outcomes of recurrent Clostridioides difficile infection., PMID:38552897
Structural and functional insight into the interaction of Clostridioides difficile toxin B and FZD7., PMID:38308843
Establishment of a Novel Detection Platform for Clostridioides difficile Toxin Genes Based on Orthogonal CRISPR., PMID:37378559
Elucidation of bezlotoxumab binding specificity to toxin B in Clostridioides difficile., PMID:37098802
Paeniclostridium sordellii uterine infection is dependent on the estrous cycle., PMID:36409774
Receptor binding mechanisms of Clostridioides difficile toxin B and implications for therapeutics development., PMID:34862749
Structural basis for CSPG4 as a receptor for TcdB and a therapeutic target in Clostridioides difficile infection., PMID:34145250
Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection Enhances Adaptive Immunity to C difficile Toxin B., PMID:33444574
Phylogenomics of 8,839 Clostridioides difficile genomes reveals recombination-driven evolution and diversification of toxin A and B., PMID:33370413
Absence of Toxemia in Clostridioides difficile Infection: Results from Ultrasensitive Toxin Assay of Serum., PMID:33164145
Human C. difficile toxin-specific memory B cell repertoires encode poorly neutralizing antibodies., PMID:32663199
Analysis of C. difficile infection-related outcomes in European participants in the bezlotoxumab MODIFY I and II trials., PMID:32504314
Development of a new serological assay for the diagnosis of Clostridium difficile infections with prognostic value., PMID:31733265
Designed Ankyrin Repeat Protein (DARPin) Neutralizers of TcdB from Clostridium difficile Ribotype 027., PMID:31578248
Assessment of Bezlotoxumab Immunogenicity., PMID:31411386
Structure of the full-length Clostridium difficile toxin B., PMID:31308519
Rapid Detection of Clostridium difficile Toxins in Stool by Raman Spectroscopy., PMID:31279995
Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B., PMID:31233493
Vaccination against Clostridium difficile by Use of an Attenuated Salmonella enterica Serovar Typhimurium Vector (YS1646) Protects Mice from Lethal Challenge., PMID:31138615
Deletion of a 19-Amino-Acid Region in Clostridioides difficile TcdB2 Results in Spontaneous Autoprocessing and Reduced Cell Binding and Provides a Nontoxic Immunogen for Vaccination., PMID:31138612
Treatment and Prevention of Recurrent Clostridium difficile Infection with Functionalized Bovine Antibody-Enriched Whey in a Hamster Primary Infection Model., PMID:30736358
Neutralization of Clostridium difficile toxin B with VHH-Fc fusions targeting the delivery and CROPs domains., PMID:30540857
The Conserved Cys-2232 in Clostridioides difficile Toxin B Modulates Receptor Binding., PMID:30416488
Clostridium difficile Toxoid Vaccine Candidate Confers Broad Protection against a Range of Prevalent Circulating Strains in a Nonclinical Setting., PMID:29632249
Actoxumab + bezlotoxumab combination: what promise for Clostridium difficile treatment?, PMID:29534621
Bezlotoxumab for the Prevention of Clostridium difficile Recurrence., PMID:29409575
Adaptive immune response to Clostridium difficile infection: A perspective for prevention and therapy., PMID:29272036
Functional defects in Clostridium difficile TcdB toxin uptake identify CSPG4 receptor-binding determinants., PMID:28842504
Bezlotoxumab for the prevention of Clostridium difficile recurrence., PMID:28805081
Bezlotoxumab: A Novel Agent for the Prevention of Recurrent Clostridium difficile Infection., PMID:28730660
Bezlotoxumab: anti-toxin B monoclonal antibody to prevent recurrence of Clostridium difficile infection., PMID:28636484
Bezlotoxumab: Could This be the Answer for Clostridium difficile Recurrence?, PMID:28480750
Bezlotoxumab: First Global Approval., PMID:27905086
Colon-Targeted Delivery of IgY Against Clostridium difficile Toxin A and B by Encapsulation in Chitosan-Ca Pectinate Microbeads., PMID:27826799
A DNA vaccine targeting TcdA and TcdB induces protective immunity against Clostridium difficile., PMID:27770789
Coordination between T helper cells, iNKT cells, and their follicular helper subsets in the humoral immune response against Clostridium difficile toxin B., PMID:27566831
Disease Progression and Resolution in Rodent Models of Clostridium difficile Infection and Impact of Antitoxin Antibodies and Vancomycin., PMID:27527088
Intravenous adenovirus expressing a multi-specific, single-domain antibody neutralizing TcdA and TcdB protects mice from Clostridium difficile infection., PMID:27502696
A Tetraspecific VHH-Based Neutralizing Antibody Modifies Disease Outcome in Three Animal Models of Clostridium difficile Infection., PMID:27413067
Antibodies to Toxin B Are Protective Against Clostridium difficile Infection Recurrence., PMID:27365387
Novel Clostridium difficile Anti-Toxin (TcdA and TcdB) Humanized Monoclonal Antibodies Demonstrate In Vitro Neutralization across a Broad Spectrum of Clinical Strains and In Vivo Potency in a Hamster Spore Challenge Model., PMID:27336843
Production and Characterization of Chemically Inactivated Genetically Engineered Clostridium difficile Toxoids., PMID:27233688
Safety, immunogenicity and dose response of VLA84, a new vaccine candidate against Clostridium difficile, in healthy volunteers., PMID:27079932
Sensitive assays enable detection of serum IgG antibodies against Clostridium difficile toxin A and toxin B in healthy subjects and patients with Clostridium difficile infection., PMID:26964649