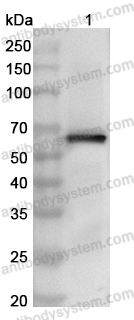
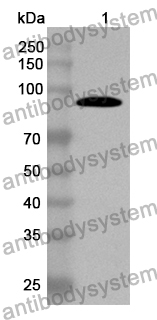
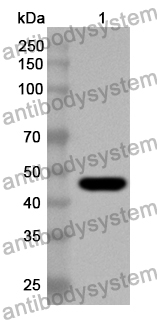
Catalog No.
PVV28802
Species reactivity
Porcine epidemic diarrhea virus (strain CV777) (PEDV)
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant PEDV N/Nucleoprotein (Met1-Asn441).
Tested applications
ELISA: 1:4000-1:8000, IHC: 1:50-1:200, IF: 1:50-1:200, WB: 1:500-1:4000
Target
Nucleoprotein, Nucleocapsid protein, NC, Protein N, N, 6
Concentration
0.85 mg/ml
Purification
Purified by antigen affinity column.
Accession
Q07499
Applications
ELISA, IHC, IF, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Transcriptomic Profiling of Porcine Duodenal, Jejunal, and Ileal Organoids in Response to Porcine Epidemic Diarrhea Virus. [PVV28802]
Transcriptomic Profiling of Porcine Duodenal, Jejunal, and Ileal Organoids in Response to Porcine Epidemic Diarrhea Virus
First study to describe the prevalence and epidemiology of African swine fever, classical swine fever, porcine reproductive and respiratory syndrome and swine flu in Kazakhstan., PMID:40437487
Teclistamab Dosing in Responders: Modeling and Simulation Results from the MajesTEC-1 Study in Relapsed/Refractory Multiple Myeloma., PMID:40332715
Development of blocking ELISA for detection anti-PRRSV antibodies and serological investigation of PRRSV in China., PMID:39793838
A Global Collaborative Comparison of SARS-CoV-2 Antigenicity Across 15 Laboratories., PMID:39772242
Protein nanoparticle vaccines induce potent neutralizing antibody responses against MERS-CoV., PMID:39644492
Pioneering molecular screening for cervical precursor lesions and cervical cancer in sera., PMID:39610929
Dynamics of Antibody Binding and Neutralization during Viral Infection., PMID:39609321
AIntibody: an experimentally validated in silico antibody discovery design challenge., PMID:39496931
Isolation and escape mapping of broadly neutralizing antibodies against emerging delta-coronaviruses., PMID:39488210
CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors., PMID:39385035
A potent pan-sarbecovirus neutralizing antibody resilient to epitope diversification., PMID:39383863
Potent neutralization of SARS-CoV-2 variants by RBD nanoparticle and prefusion-stabilized spike immunogens., PMID:39379400
Molecular imaging supports the development of multispecific cancer antibodies., PMID:39327536
Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: a phase 2 adaptive trial., PMID:39284953
Multistage protective anti-CelTOS monoclonal antibodies with cross-species sterile protection against malaria., PMID:39209843
Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals., PMID:39197450
Efficacy and safety of Butantan-DV in participants aged 2-59 years through an extended follow-up: results from a double-blind, randomised, placebo-controlled, phase 3, multicentre trial in Brazil., PMID:39116904
Computationally designed mRNA-launched protein nanoparticle vaccines., PMID:39091730
Similar Limited Protection Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Infection in Vaccinated Individuals With HIV and Comparable Controls., PMID:39070044
Luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): primary analysis of a phase 3, open-label, randomised, controlled trial., PMID:39038479
A broadly generalizable stabilization strategy for sarbecovirus fusion machinery vaccines., PMID:38944664
The Effect of Exposure to SARS-CoV-2 Vaccination and Infection on Humoral and Cellular Immunity in a Cohort of Patients with Immune-Mediated Diseases: A Pilot Study., PMID:38921803
Mono- and Bi-specific Nanobodies Targeting the CUB Domains of PCPE-1 Reduce the Proteolytic Processing of Fibrillar Procollagens., PMID:38901640
A pre-vaccination immune metabolic interplay determines the protective antibody response to a dengue virus vaccine., PMID:38900640
Anti-HIV Humoral Response Induced by Different Anti-Idiotype Antibody Formats: An In Silico and In Vivo Approach., PMID:38891926
NRG-BN002: Phase I study of ipilimumab, nivolumab, and the combination in patients with newly diagnosed glioblastoma., PMID:38874333
Opposing effects of pre-existing antibody and memory T cell help on the dynamics of recall germinal centers., PMID:38838672
Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study., PMID:38830991
Dynamics of antibody binding and neutralization during viral infection., PMID:38800656
Immunogenicity of a bivalent BA.1 COVID-19 booster vaccine in people with HIV in the Netherlands., PMID:38788210
Innate Responses to the Former COVID-19 Vaccine Candidate CVnCoV and Their Relation to Reactogenicity and Adaptive Immunogenicity., PMID:38675770
Correlation of immune fitness with response to teclistamab in relapsed/refractory multiple myeloma in the MajesTEC-1 study., PMID:38657201
Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans., PMID:38490197
Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals., PMID:38370696
Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor., PMID:38337221
Live, Attenuated, Tetravalent Butantan-Dengue Vaccine in Children and Adults., PMID:38294972
Improving stroke outcomes in hyperglycemic mice by modulating tPA/NMDAR signaling to reduce inflammation and hemorrhages., PMID:38190586
A broadly generalizable stabilization strategy for sarbecovirus fusion machinery vaccines., PMID:38168207
Biophysical studies do not reveal direct interactions between human PF4 and Ad26.COV2.S vaccine., PMID:38159648
Identification of highly selective SIK1/2 inhibitors that modulate innate immune activation and suppress intestinal inflammation., PMID:38147543
Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma., PMID:38084760
Durable immunity to SARS-CoV-2 in both lower and upper airways achieved with a gorilla adenovirus (GRAd) S-2P vaccine in non-human primates., PMID:38076895
Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: importance of immunoglobulin supplementation., PMID:38052042
Broad receptor tropism and immunogenicity of a clade 3 sarbecovirus., PMID:37989312
Circulating tumor DNA landscape and prognostic impact of acquired resistance to targeted therapies in cancer patients: a national center for precision medicine (PRISM) study., PMID:37924050
Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:37919269
Recommendations for the Clinical Approach to Immune Thrombocytopenia: Spanish ITP Working Group (GEPTI)., PMID:37892566
Safety and Immunogenicity of Inactivated Whole Virion COVID-19 Vaccine CoviVac in Clinical Trials in 18-60 and 60+ Age Cohorts., PMID:37766235
Broad receptor tropism and immunogenicity of a clade 3 sarbecovirus., PMID:37745523
Neutralization, effector function and immune imprinting of Omicron variants., PMID:37648855