Catalog No.
PAC90703
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
Ocrelizumab
Tested applications
ELISA: 1:4000-1:8000
Target
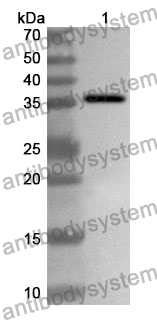
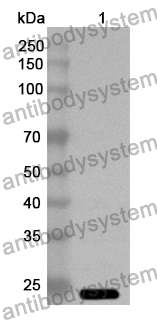
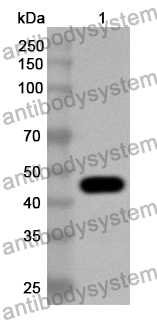
Rabbit polyclonal to Ocrevus.
Specificity
The product is specific for Ocrelizumab. This antibody serves as an excellent positive control for Ocrelizumab immunogenicity (ADA) assays.
Concentration
0.18 mg/ml
Purification
Purified by antigen affinity column.
Accession
CAS: 637334-45-3
Applications
ELISA
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
The product can be stored for 2 - 3 weeks at 2 to 8°C or for up to 12 months at -20°C. Avoid repeated freezing and thawing cycles.
Background
Ocrelizumab (Ocrevus®) is an intravenously administered, humanized anti-CD20 monoclonal antibody approved for the treatment of adults with relapsing forms of multiple sclerosis (RMS) or primary progressive multiple sclerosis (PPMS). Antibodysystem Anti-Ocrelizumab Antibody, pAb, Rabbit is produced from sera of a rabbit immunized with Ocrelizumab.
Role of Ocrelizumab in modulating gene and microRNA expression in multiple sclerosis., PMID:40510879
What Is the Evidence on Immunomodulators and Immunosuppressants for Progressive Multiple Sclerosis? - A Cochrane Review Summary with Commentary., PMID:40485316
CONFIDENCE treatment success: long-term real-world effectiveness and safety of ocrelizumab in Germany., PMID:40470489
A disproportionality analysis of nervous system adverse events associated with disease-modifying therapies in multiple sclerosis: insights from the FDA adverse event reporting system (FAERS)., PMID:40465056
Comparative effectiveness of natalizumab and anti-CD20 monoclonal antibodies in relapsing-remitting multiple sclerosis: a real-world propensity-score matched study., PMID:40451284
Ocrelizumab-Induced Hemophagocytic Lymphohistiocytosis: A Case Report., PMID:40438805
Vaccine-Induced Humoral and Cellular Response to SARS-CoV-2 in Multiple Sclerosis Patients on Ocrelizumab., PMID:40432100
Real-world observational study of infections in people treated with ocrelizumab for multiple sclerosis., PMID:40402264
Seronegative spondyloarthritis as a complication of anti-CD20 monoclonal antibody treatment in multiple sclerosis., PMID:40367683
Comprehensive analysis of B cell repopulation in ocrelizumab-treated patients with multiple sclerosis by mass cytometry and proteomics., PMID:40322080
Severe Late-Onset Neutropenia in a Pregnant Patient with Multiple Sclerosis after Ocrelizumab., PMID:40292041
Switching to ublituximab from prior anti-CD20 monoclonal antibody therapy: a case report series., PMID:40255393
Subcutaneous Ocrelizumab in Patients With Multiple Sclerosis: Results of the Phase 3 OCARINA II Study., PMID:40245351
Short- and long-term effects of early versus delayed treatment with ocrelizumab on cerebellar volume loss in patients with RMS and PPMS., PMID:40237070
Hypogammaglobulinemia in patients with multiple sclerosis receiving disease modifying therapies: disproportionality analysis using the EudraVigilance database., PMID:40235349
Efficacy of ocrelizumab versus rituximab in patients with relapsing-remitting multiple sclerosis., PMID:40216701
Effects of Anti-CD20 Antibody Therapy on Immune Cell Dynamics in Relapsing-Remitting Multiple Sclerosis., PMID:40214505
Recall vaccination increases detectable B-cell reactivity in persons with multiple sclerosis treated with ocrelizumab., PMID:40186086
Clinical and radiological activity after extended interval and standard interval dosing of ocrelizumab in multiple sclerosis: A systematic review and meta-analysis., PMID:40183837
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function., PMID:40180777
Pyoderma gangrenosum in a patient with multiple sclerosis under natalizumab treatment: a case report., PMID:40175973
Home-sampling of B cells using quantitative dried blood spots to enable tailored therapeutic re-dosing of anti-CD20 therapies., PMID:40167038
Therapeutic Advances in Pediatric Multiple Sclerosis., PMID:40150542
No evidence of fluctuations in daily step count between infusions in people with multiple sclerosis treated with anti-CD20 monoclonal antibodies., PMID:40144903
Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs., PMID:40137720
[Late-onset neutropenia following ocrelizumab treatment in relapsing multiple sclerosis]., PMID:40079287
Effect of Treatment on Steroidome in Women with Multiple Sclerosis., PMID:40076462
Gut IgA-antibody secreting cells segregate into four Blimp1+ subsets based on differential expression of IgA and Ki-67 and are retained following prolonged αCD20 B cell depletion in mice., PMID:40073093
Rituximab for people with multiple sclerosis., PMID:40066932
[Comparing the efficacy of divozilimab and second-line treatments for relapsing-remitting multiple sclerosis in the Russian Federation: a systematic review and network meta-analysis]., PMID:40047834
Severe coxsackie virus B5 encephalitis mimics autoimmune limbic encephalitis in a young woman under long-term B-cell depletion with ocrelizumab: A case report., PMID:40012477
B-Cell and T-Cell Populations in Peripheral Blood Linked to Ocrelizumab Treatment Efficacy in Multiple Sclerosis., PMID:40010950
A disproportionality analysis of surgical site infections across multiple sclerosis disease modifying therapies., PMID:39985588
Extended-interval dosing of rituximab/ocrelizumab is associated with a reduced decrease in IgG levels in multiple sclerosis., PMID:39979176
Ocrelizumab-induced colitis-critical review and case series from a Romanian cohort of MS patients., PMID:39974366
Cognitive effects of ocrelizumab vs interferon β-1a in relapsing multiple sclerosis: A post hoc analysis of the OPERA I/II trials., PMID:39965438
Management of patients with active relapsing-remitting or secondary progressive multiple sclerosis: A French real-world study based on claims data linked to a phase IV study., PMID:39951916
Modulator of VRAC Current 1 Is a Potential Target Antigen in Multiple Sclerosis., PMID:39933126
Echovirus serotype 11 induced sepsis in a young female patient with multiple sclerosis treated with anti-CD20 monoclonal antibody ocrelizumab., PMID:39907911
Long-Term Treatment With Ocrelizumab in Patients With Early-Stage Relapsing MS: Nine-Year Data From the OPERA Studies Open-Label Extension., PMID:39883906
Comparative effectiveness, safety and persistence of ocrelizumab versus natalizumab in multiple sclerosis: A real-world, multi-center, propensity score-matched study., PMID:39880749
Safety and Efficacy of Fingolimod and Ocrelizumab in Pediatric Patients With Multiple Sclerosis., PMID:39879675
Effectiveness of Ocrelizumab on Disease Progression and Disability Status in Multiple Sclerosis Patients: A Two-Year Prospective Cohort Study., PMID:39860559
Effect of Different Treatments on Retinal Thickness Changes in Patients With Multiple Sclerosis: A Review., PMID:39853938
Clinical and analytical monitoring of patients with multiple sclerosis on anti-CD20 therapeutics: a real-world safety profile study., PMID:39835156
Ocrelizumab dose selection for treatment of pediatric relapsing-remitting multiple sclerosis: results of the OPERETTA I study., PMID:39812825
Comparing the efficacy and safety of extended vs standard dosing of ocrelizumab in MS: A systemic review and meta-analysis., PMID:39805179
Assessment of dysphagia and its associations in patients with secondary progressive multiple sclerosis., PMID:39793523
Disseminated enterovirus infection treated with intravenous immunoglobulin in a patient on maintenance ocrelizumab for multiple sclerosis., PMID:39778949
Real-world evidence of ocrelizumab in Chilean patients with multiple sclerosis., PMID:39757941