Catalog No.
KAH02251
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Pembrolizumab has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled Human CD279 antigen and then pipetted into the wells. Anti-Pembrolizumab Neutralizing Antibody in the sample competitively binds to Pembrolizumab with the biotin-labeled Human CD279 antigen. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anti-Pembrolizumab Neutralizing Antibody bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Pembrolizumab Neutralizing Antibody concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
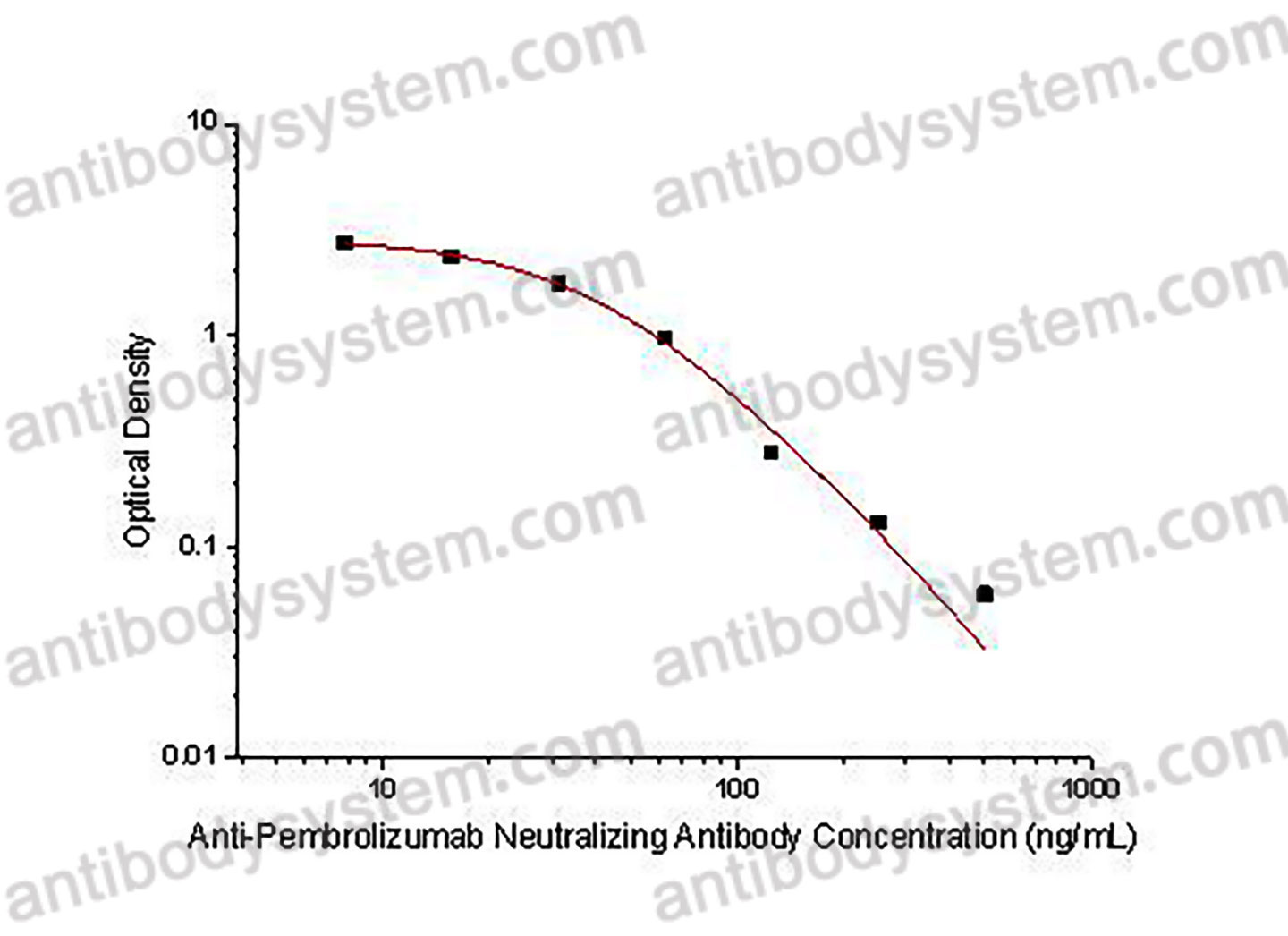
Range
7.81 - 500 ng/mL
Sensitivity
6.90 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
292.6
|
78.7
|
19.0
|
293.1
|
75.5
|
21.7
|
|
Standard deviation
|
15.7
|
7.4
|
3.7
|
27.1
|
4.2
|
3.3
|
|
CV (%)
|
5.4
|
9.3
|
19.5
|
9.2
|
5.6
|
15.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MK-3475, CAS: 1374853-91-4
Which test results to believe? Comparison of different ELISA kits for detection of SARS-CoV-2-neutralizing antibody among COVID-vaccinated individuals., PMID:40463604
Detection of antibodies against infectious bronchitis virus strain QX (GI-19) by commercial ELISA kits and virus neutralization test., PMID:40456450
Detection and Comparison of Sow Serum Samples from Herds Regularly Mass Vaccinated with Porcine Reproductive and Respiratory Syndrome Modified Live Virus Using Four Commercial Enzyme-Linked Immunosorbent Assays and Neutralizing Tests., PMID:40431595
mRNA COVID-19 vaccines induce superior IgA titers in cancer patients compared to viral vector vaccines: Implications for immunization strategies., PMID:40414552
Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains., PMID:40333311
Longitudinal Follow-Up of the Specific Antibody Response to SARS-CoV-2 Vaccination in Colombia., PMID:39817585
Anti-SARS-CoV-2 total immunoglobulin and neutralising antibody responses in healthy blood donors throughout the COVID-19 pandemic: a longitudinal observational study., PMID:39137369
Efficient transplacental transfer of SARS-CoV-2 antibodies between naturally exposed mothers and infants in Accra, Ghana., PMID:38730052
Whole Blood as a Sample Matrix in Homogeneous Time-Resolved Assay-Förster Resonance Energy Transfer-Based Antibody Detection., PMID:38611633
Elevated CD4+ T Cell Senescence Associates with Impaired Immune Responsiveness in Severe COVID-19., PMID:38377029
[Bovine viral diarrhea virus Erns protein expressed in Chinese hamster ovary cells and its immunogenicity analysis]., PMID:38147987
Potent immunogenicity and neutralization of recombinant adeno-associated virus expressing the glycoprotein of severe fever with thrombocytopenia virus., PMID:38143087
Characterization of SARS-CoV-2-specific humoral immunity and associated factors in the healthy population post-vaccination., PMID:38103966
Cold-inducible RNA-binding protein induces inflammatory responses via NF-κB signaling pathway in normal human bronchial epithelial cells infected with streptococcus pneumoniae., PMID:38064816
A retrospective study of SARS-CoV-2 seroprevalence in dogs and cats in the Community of Madrid, Spain., PMID:37869682
Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern., PMID:37834413
Development and Characterization of Guinea Pig Anti-Insulin Polyclonal Antibody., PMID:37713062
Development of ELISA-Based Assay for Detection of SARS-CoV-2 Neutralizing Antibody., PMID:37643285
Electrogenic Staphylococcus epidermidis colonizes nasal cavities and alleviates IL-6 progression induced by the SARS2-CoV nucleocapsid protein., PMID:37558389
Analytical and Clinical Performance of Two Point of Care Rapid Antibody Assays for SARS-CoV-2., PMID:37437941
Clinical characteristics and host immunity responses of SARS-CoV-2 Omicron variant BA.2 with deletion of ORF7a, ORF7b and ORF8., PMID:37248496
Handheld NIR-to-NIR Platform for on-site evaluating protective neutralizing antibody against SARS-CoV-2 ancestral strain and Omicron variant after vaccination or infection., PMID:37120945
Performance evaluation of newly developed surrogate virus neutralization tests for detecting neutralizing antibodies against SARS-CoV-2., PMID:36973368
Field Performance of a Rapid Test to Detect Progressive, Regressive, and Abortive Feline Leukemia Virus Infections in Domestic Cats in Australia and Germany., PMID:36851705
Development and application of an indirect ELISA for the serological detection of bovine viral diarrhea virus infection based on the protein E2 antigen., PMID:36849860
Evaluation of a biotin-based surrogate virus neutralization test for detecting postvaccination antibodies against SARS-CoV-2 variants in sera., PMID:36696754
SARS-CoV-2 delta (B.1.617.2) spike protein adjuvanted with Alum-3M-052 enhances antibody production and neutralization ability., PMID:36684881
A highly sensitive bead-based flow cytometric competitive binding assay to detect SARS-CoV-2 neutralizing antibody activity., PMID:36532082
[The regulatory function of tumor-infiltrating Th9 cells to anti-tumor activity of CD8(+) T cells in patients with gastric cancer]., PMID:36380667
Serological Immune Response Following ChAdOx1 nCoV-19 Vaccine (Covishield®) in Patients with Liver Cirrhosis., PMID:36366346
A Novel Dry-Stabilized Whole Blood Microsampling and Protein Extraction Method for Testing of SARS-CoV-2 Antibody Titers., PMID:36298625
Development and Evaluation of a Rapid Neutralizing Antibody Assay for COVID-19 Vaccination., PMID:36278077
Antibody profile in post-vaccinated & SARS-CoV-2 infected individuals., PMID:36124500
Factors influencing neutralizing antibody titers elicited by coronavirus disease 2019 vaccines., PMID:36096357
Safety and immunogenicity of a recombinant receptor-binding domain-based protein subunit vaccine (Noora vaccine™) against COVID-19 in adults: A randomized, double-blind, placebo-controlled, Phase 1 trial., PMID:36029105
Low Measles Seropositivity Rate among Thai Adolescents in the Thai National Immunization Program., PMID:36016157
Efficacy of Sinopharm® COVID-19 Vaccine in Hemodialysis Patients: A Preliminary Report., PMID:35962641
Seroprevalence of Zika Virus among Forest Fringe Communities in Peninsular Malaysia and Sabah: General Population-Based Study., PMID:35895331
Evaluation of response to different COVID-19 vaccines in vaccinated healthcare workers in a single center in Iran., PMID:35883215
Development and characterization of anti-HPV16 monoclonal antibodies for assembly of an HPV16 detection kit., PMID:35841266
Construction of ratiometric Si-Mn:ZnSe nanoparticles for the immunoassay of SARS-CoV-2 spike protein., PMID:35813462
Seroprevalence of chikungunya virus among military personnel in Papua New Guinea, 2019., PMID:35755470
High Incidence of SARS-CoV-2 Variant of Concern Breakthrough Infections Despite Residual Humoral and Cellular Immunity Induced by BNT162b2 Vaccination in Healthcare Workers: A Long-Term Follow-Up Study in Belgium., PMID:35746728
Artificial intelligence-assisted colorimetric lateral flow immunoassay for sensitive and quantitative detection of COVID-19 neutralizing antibody., PMID:35696869
Immunogenicity of Inactivated SARS-CoV-2 Vaccines in Patients With Rheumatoid Arthritis: A Case Series., PMID:35548080
Evaluation of R-FIND SARS-CoV-2 Neutralizing Antibody ELISA and FREND COVID-19 SP Rapid Fluorescence Immunoassay., PMID:35536090
Development of HPV58 type-specific antibodies and detection kit., PMID:35465823
Efficacy and Safety of Sinopharm Vaccine for SARS-CoV-2 and breakthrough infections in Iranian Patients with Hemoglobinopathies: A Preliminary Report., PMID:35444764
Anti-SARS-CoV-2 Neutralizing Antibody Responses after Two Doses of ChAdOx1 nCoV-19 vaccine (AZD1222) in Healthcare Workers., PMID:35384425
Anti-SARS-CoV-2 IgG and Neutralizing Antibody Levels in Patients with Past COVID-19 Infection: A Longitudinal Study., PMID:35378574