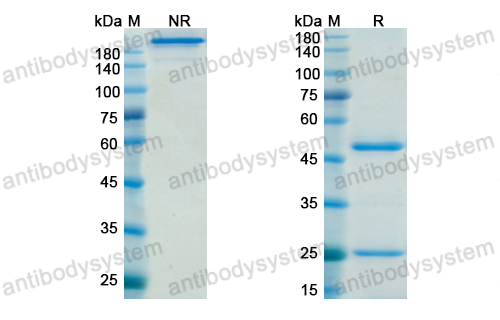
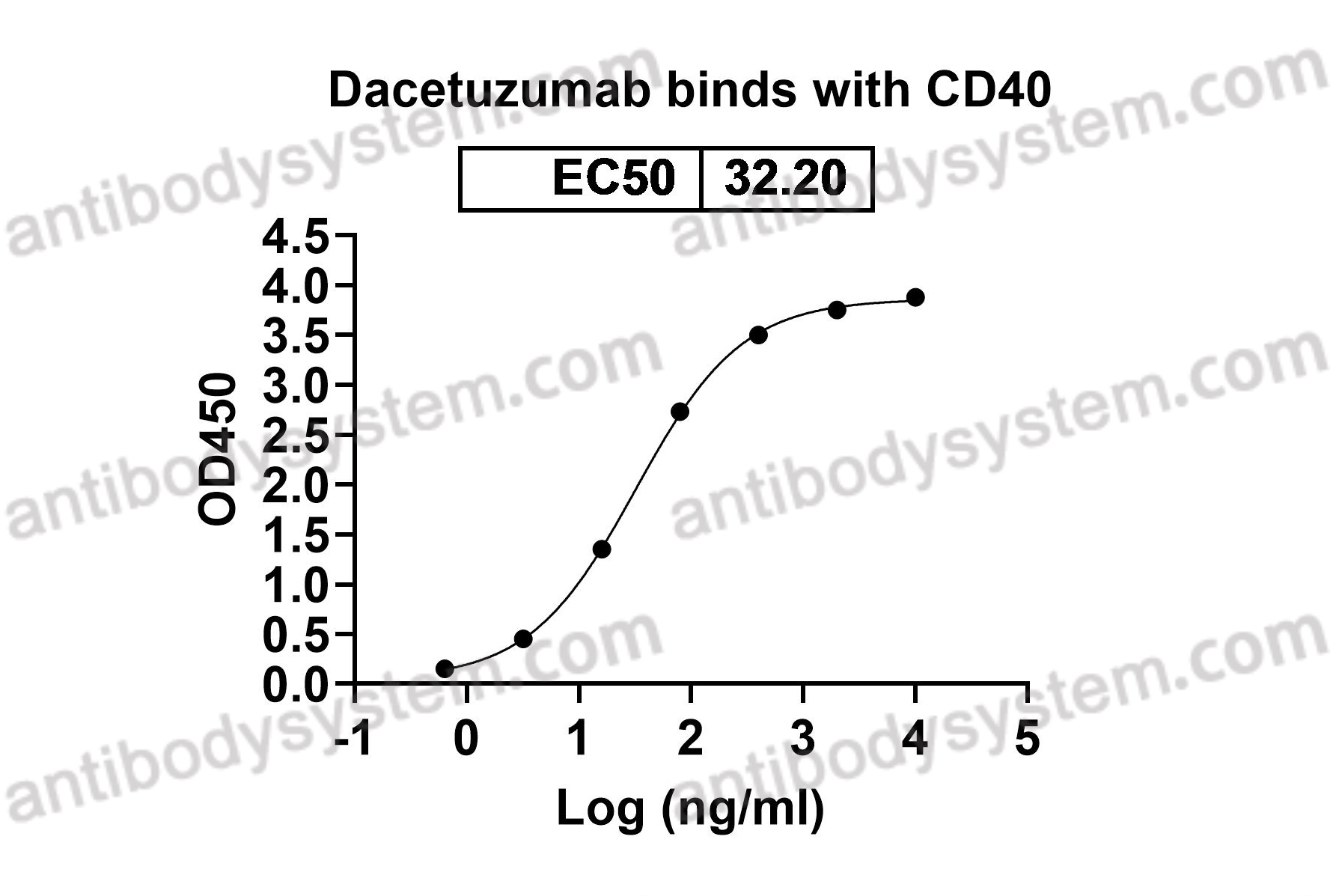
Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial, PMID: 25651427
A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma, PMID: 20133895
Pilot study of dacetuzumab in combination with rituximab and gemcitabine for relapsed or refractory diffuse large B-cell lymphoma, PMID: 22775314
Distinct apoptotic signaling characteristics of the anti-CD40 monoclonal antibody dacetuzumab and rituximab produce enhanced antitumor activity in non-Hodgkin lymphoma, PMID: 21610152
Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma, PMID: 19636010
Proapoptotic signaling activity of the anti-CD40 monoclonal antibody dacetuzumab circumvents multiple oncogenic transformation events and chemosensitizes NHL cells, PMID: 21394099
A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors, PMID: 24919462
A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia, PMID: 20038235
Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies, PMID: 19513947
Network meta-analysis of targeted therapies for diffuse large B cell lymphoma, PMID: 33308179
ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4), PMID: 29572070
Innovative Therapeutic Approaches in Primary Cutaneous B Cell Lymphoma, PMID: 32850331
Emerging antibodies for the treatment of multiple myeloma, PMID: 27195659
Emerging immune targets for the treatment of multiple myeloma, PMID: 29421983
Immunotherapy of cancer in 2012, PMID: 22576456
Anti-CD40-mediated cancer immunotherapy: an update of recent and ongoing clinical trials, PMID: 24555495
Investigational Monoclonal Antibodies in the Treatment of Multiple Myeloma: A Systematic Review of Agents under Clinical Development, PMID: 31544840
Humoral theory of transplantation: some hot topics, PMID: 23396319
Gateways to clinical trials, PMID: 19357798
Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art, PMID: 25249370
Gateways to clinical trials, PMID: 20140276
Beyond rituximab: The future of monoclonal antibodies in B-cell non-Hodgkin lymphoma, PMID: 20425465
Updating targets for natural killer/T-cell lymphoma immunotherapy, PMID: 33628584
[Therapeutic monoclonal antibodies against multiple myeloma], PMID: 25626316
CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma, PMID: 21411738
Statistical techniques to construct assays for identifying likely responders to a treatment under evaluation from cell line genomic data, PMID: 20979617
Primary biliary cholangitis: a summary of pathogenesis and therapies., PMID:40124425
Crystal structures of human CD40 in complex with monoclonal antibodies dacetuzumab and bleselumab., PMID:38657446
Updating targets for natural killer/T-cell lymphoma immunotherapy., PMID:33628584
Network meta-analysis of targeted therapies for diffuse large B cell lymphoma., PMID:33308179
Innovative Therapeutic Approaches in Primary Cutaneous B Cell Lymphoma., PMID:32850331
Investigational Monoclonal Antibodies in the Treatment of Multiple Myeloma: A Systematic Review of Agents under Clinical Development., PMID:31544840
ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4)., PMID:29572070
Emerging immune targets for the treatment of multiple myeloma., PMID:29421983
Emerging antibodies for the treatment of multiple myeloma., PMID:27195659
Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial., PMID:25651427
[Therapeutic monoclonal antibodies against multiple myeloma]., PMID:25626316
Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art., PMID:25249370
A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors., PMID:24919462
Anti-CD40-mediated cancer immunotherapy: an update of recent and ongoing clinical trials., PMID:24555495
Humoral theory of transplantation: some hot topics., PMID:23396319
Pilot study of dacetuzumab in combination with rituximab and gemcitabine for relapsed or refractory diffuse large B-cell lymphoma., PMID:22775314
Immunotherapy of cancer in 2012., PMID:22576456
Distinct apoptotic signaling characteristics of the anti-CD40 monoclonal antibody dacetuzumab and rituximab produce enhanced antitumor activity in non-Hodgkin lymphoma., PMID:21610152
CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma., PMID:21411738
Proapoptotic signaling activity of the anti-CD40 monoclonal antibody dacetuzumab circumvents multiple oncogenic transformation events and chemosensitizes NHL cells., PMID:21394099
Statistical techniques to construct assays for identifying likely responders to a treatment under evaluation from cell line genomic data., PMID:20979617
Gateways to clinical trials., PMID:20140276
A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma., PMID:20133895
A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia., PMID:20038235
Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma., PMID:19636010
Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies., PMID:19513947
Gateways to clinical trials., PMID:19357798
Beyond rituximab: The future of monoclonal antibodies in B-cell non-Hodgkin lymphoma., PMID:20425465