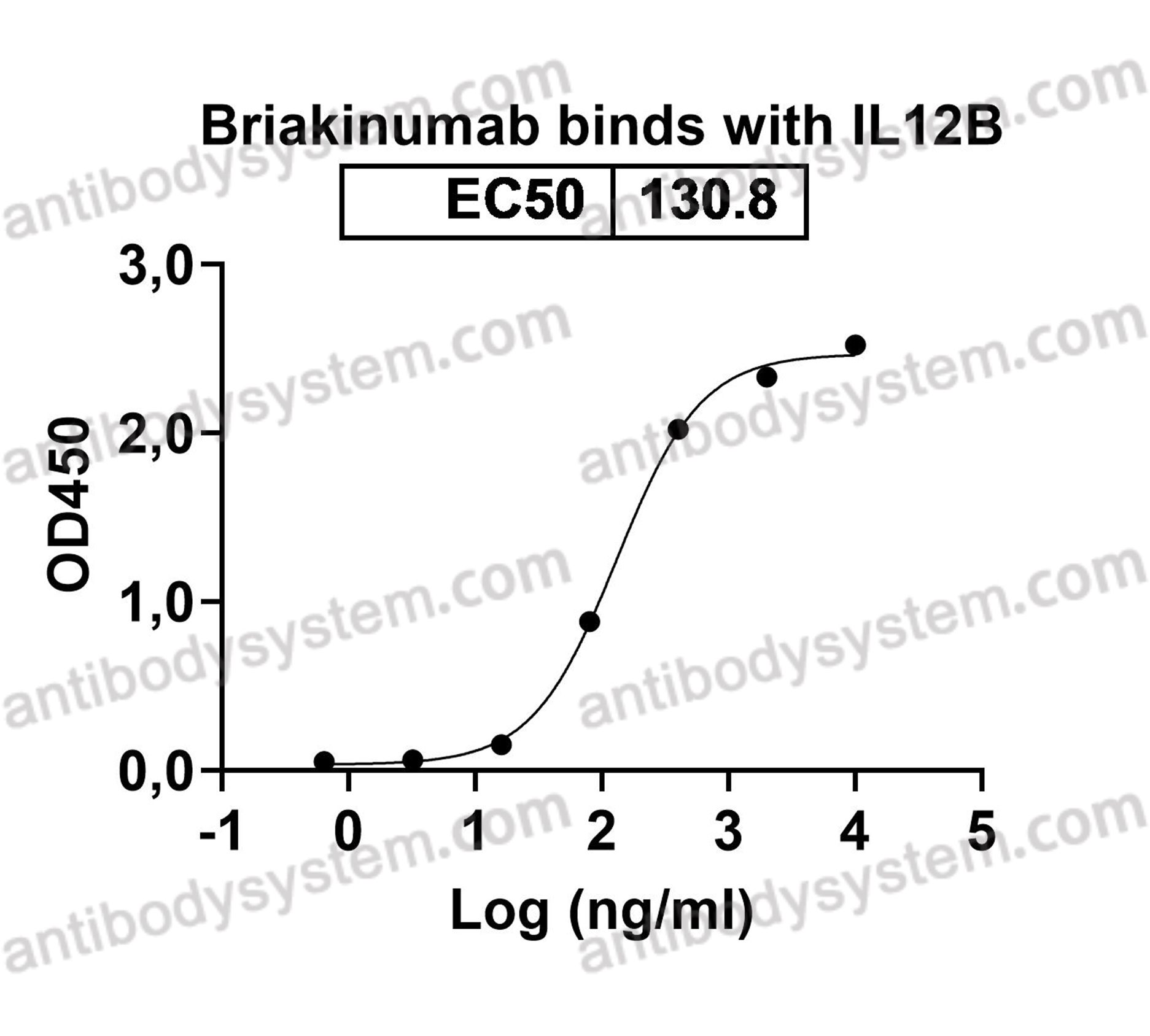
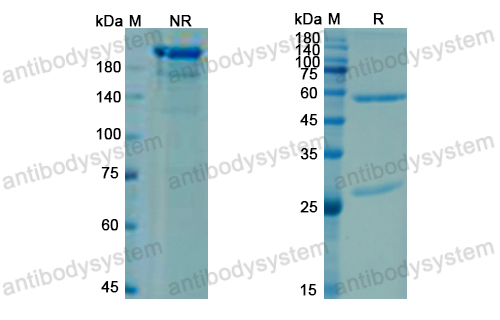
A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis, PMID: 22029980
A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis, PMID: 22011907
A Review of Biologic Therapies Targeting IL-23 and IL-17 for Use in Moderate-to-Severe Plaque Psoriasis, PMID: 26714681
A Two-pronged Binding Mechanism of IgG to the Neonatal Fc Receptor Controls Complex Stability and IgG Serum Half-life, PMID: 28062799
ABT-874, a fully human monoclonal anti-IL-12/IL-23 antibody for the potential treatment of autoimmune diseases, PMID: 18465662
Antibodies in the Treatment of Psoriasis: IL-12/23 p40 and IL-17a, PMID: 27551698
Antibody-based therapeutics to watch in 2011, PMID: 21051951
Anti-IL-12/23 in Crohn's disease: bench and bedside, PMID: 24138637
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease, PMID: 25942580
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease, PMID: 27885650
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease, PMID: 31828765
Anti-p40 antibodies ustekinumab and briakinumab: blockade of interleukin-12 and interleukin-23 in the treatment of psoriasis, PMID: 20430307
Association of anti-IL-12/23 biologic agents ustekinumab and briakinumab with major adverse cardiovascular events, PMID: 23461311
Association of ustekinumab and briakinumab with major adverse cardiovascular events: An appraisal of meta-analyses and industry sponsored pooled analyses to date, PMID: 23467502
Briakinumab for the treatment of plaque psoriasis, PMID: 22077474
Briakinumab for treatment of Crohn's disease: results of a randomized trial, PMID: 25989338
Briakinumab versus methotrexate for psoriasis, PMID: 22276832
Briakinumab versus Methotrexate for Psoriasis, PMID: 22276833
Briakinumab, PMID: 19569977
Cardiovascular risk and psoriasis: the role of biologic therapy, PMID: 23157913
Comparative efficacy and safety of thirteen biologic therapies for patients with moderate or severe psoriasis: A network meta-analysis, PMID: 30922656
Could anti IL12/23 therapy replace anti-TNF biologics?, PMID: 19818214
Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents, PMID: 20590580
Differential response of chronic plaque psoriasis to briakinumab vs. ustekinumab, PMID: 22169986
Does p40-targeted therapy represent a significant evolution in the management of plaque psoriasis?, PMID: 22758911
Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study, PMID: 23679896
Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis, PMID: 21574983
Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis, PMID: 21574984
Efficacy of several biological therapies for treating moderate to severe psoriasis: A network meta-analysis, PMID: 30546410
Emerging Treatment Options in Inflammatory Bowel Disease: Janus Kinases, Stem Cells, and More, PMID: 32570252
Immunotherapy in inflammatory bowel disease, PMID: 22703854
Interleukin-23 and interleukin-17: importance in pathogenesis and therapy of psoriasis, PMID: 23122008
Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases, PMID: 27780712
New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab, PMID: 21348542
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID: 28765121
Overview of biologic therapy for Crohn's disease, PMID: 19591627
Psoriasis and cardiovascular comorbidities with emphasis in Asia, PMID: 22481582
Psoriasis and cardiovascular disorders, PMID: 22481581
Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis, PMID: 23157612
Specific targeting of interleukin-23p19 as effective treatment for psoriasis, PMID: 24373779
Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rβ1, PMID: 29287995
Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab, PMID: 24800703
Target-independent variable region mediated effects on antibody clearance can be FcRn independent, PMID: 27610650
Targeting the interleukin-12/23 cytokine family in the treatment of psoriatic disease, PMID: 19886505
Th17 cells: a new therapeutic target in inflammatory dermatoses, PMID: 18626814
The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases, PMID: 22280236
The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade, PMID: 28541488
Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases, PMID: 26565676
Tildrakizumab: A Review of Phase II and III Clinical Trials, PMID: 30345790
Treatment of nail psoriasis with TNF-α or IL12/23 inhibitors, PMID: 22859238
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Prioritizing drug targets in systemic lupus erythematosus from a genetic perspective: a druggable genome-wide Mendelian randomization study., PMID:38997544
IL-12 and IL-23 pathway inhibition in inflammatory bowel disease., PMID:37069321
Adverse Effects of Anti-Interleukin-23 Agents Employed in Patients with Psoriasis: A Systematic Review., PMID:35697004
Cardiovascular Safety of Biologics Targeting Interleukin (IL)-12 and/or IL-23: What Does the Evidence Say?, PMID:34292509
Emerging Treatment Options in Inflammatory Bowel Disease: Janus Kinases, Stem Cells, and More., PMID:32570252
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease., PMID:31828765
Comparative efficacy and safety of thirteen biologic therapies for patients with moderate or severe psoriasis: A network meta-analysis., PMID:30922656
Efficacy of several biological therapies for treating moderate to severe psoriasis: A network meta-analysis., PMID:30546410
Tildrakizumab: A Review of Phase II and III Clinical Trials., PMID:30345790
Inflammatory bowel disease therapy: blockade of cytokines and cytokine signaling pathways., PMID:29846261
Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rβ1., PMID:29287995
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID:28765121
The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade., PMID:28541488
A Two-pronged Binding Mechanism of IgG to the Neonatal Fc Receptor Controls Complex Stability and IgG Serum Half-life., PMID:28062799
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:27885650
Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases., PMID:27780712
Target-independent variable region mediated effects on antibody clearance can be FcRn independent., PMID:27610650
Antibodies in the Treatment of Psoriasis: IL-12/23 p40 and IL-17a., PMID:27551698
A Review of Biologic Therapies Targeting IL-23 and IL-17 for Use in Moderate-to-Severe Plaque Psoriasis., PMID:26714681
Variation at NRG1 genotype related to modulation of small-world properties of the functional cortical network., PMID:26650688
Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases., PMID:26565676
The IL-12/23/STAT Axis as a Therapeutic Target in Inflammatory Bowel Disease: Mechanisms and Evidence in Man., PMID:26366705
Briakinumab for treatment of Crohn's disease: results of a randomized trial., PMID:25989338
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:25942580
Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics., PMID:25918417
Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab., PMID:24800703
Specific targeting of interleukin-23p19 as effective treatment for psoriasis., PMID:24373779
Anti-IL-12/23 in Crohn's disease: bench and bedside., PMID:24138637
Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study., PMID:23679896
Major cardiovascular events associated with anti-IL 12/23 agents: a tale of two meta-analyses., PMID:23602173
Association of ustekinumab and briakinumab with major adverse cardiovascular events: An appraisal of meta-analyses and industry sponsored pooled analyses to date., PMID:23467502
Association of anti-IL-12/23 biologic agents ustekinumab and briakinumab with major adverse cardiovascular events., PMID:23461311
Male and female fertility assessment in the cynomolgus monkey following administration of ABT-874, a human Anti-IL-12/23p40 monoclonal antibody., PMID:23213064
Pre- and postnatal development in the cynomolgus monkey following administration of ABT-874, a human anti-IL-12/23p40 monoclonal antibody., PMID:23212752
Cardiovascular risk and psoriasis: the role of biologic therapy., PMID:23157913
Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis., PMID:23157612
Interleukin-23 and interleukin-17: importance in pathogenesis and therapy of psoriasis., PMID:23122008
Treatment of nail psoriasis with TNF-α or IL12/23 inhibitors., PMID:22859238
Does p40-targeted therapy represent a significant evolution in the management of plaque psoriasis?, PMID:22758911
Immunotherapy in inflammatory bowel disease., PMID:22703854
Cardiovascular Risk and Psoriasis: the Role of Biologic Therapy., PMID:22591655
Psoriasis and cardiovascular comorbidities with emphasis in Asia., PMID:22481582
Psoriasis and cardiovascular disorders., PMID:22481581
Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials., PMID:22404103
The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases., PMID:22280236
Briakinumab versus Methotrexate for Psoriasis., PMID:22276833
Briakinumab versus methotrexate for psoriasis., PMID:22276832
Differential response of chronic plaque psoriasis to briakinumab vs. ustekinumab., PMID:22169986
Briakinumab for the treatment of plaque psoriasis., PMID:22077474