Catalog No.
DHC85101
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Mouse
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CD37, Tspan-26, TSPAN26, Leukocyte antigen CD37, Tetraspanin-26
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
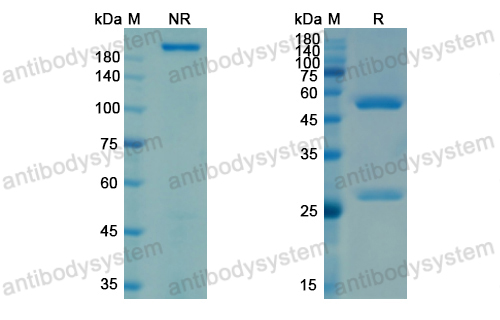
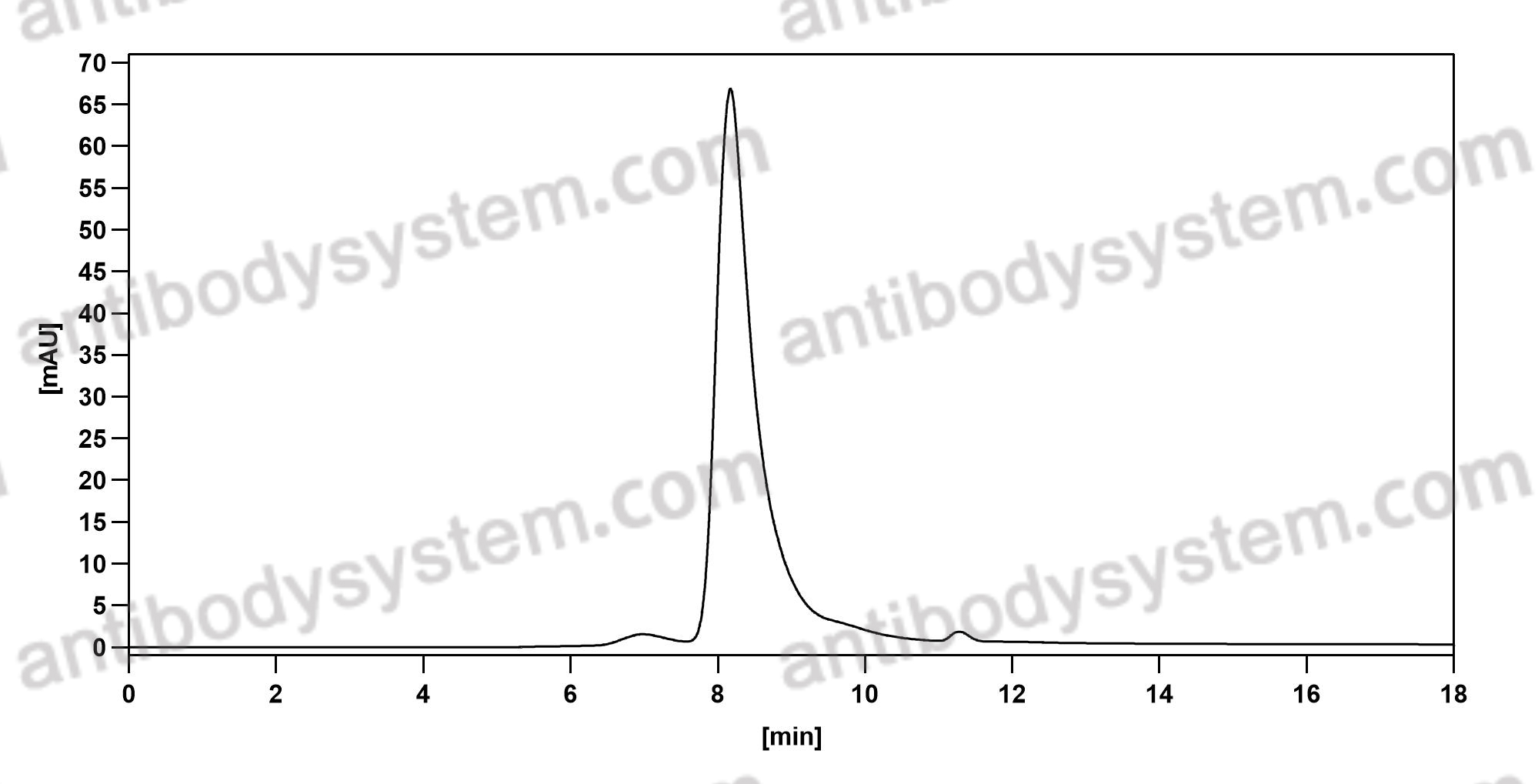
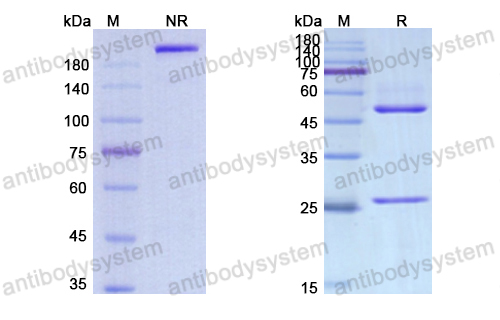
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P11049
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
HH1, CAS: 1453362-55-4
Clone ID
Lilotomab
The therapeutic effectiveness of 177 Lu-lilotomab in B-cell non-Hodgkin lymphoma involves modulation of G2/M cell cycle arrest, PMID: 31836849
177 Lu-Lilotomab Satetraxetan Has the Potential to Counteract Resistance to Rituximab in Non-Hodgkin Lymphoma, PMID: 32245896
Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine, PMID: 31481583
Combination of 177 Lu-lilotomab with rituximab significantly improves the therapeutic outcome in preclinical models of non-Hodgkin's lymphoma, PMID: 29993152
Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma, PMID: 32877524
Pre-dosing with lilotomab prior to therapy with 177 Lu-lilotomab satetraxetan significantly increases the ratio of tumor to red marrow absorbed dose in non-Hodgkin lymphoma patients, PMID: 29470615
FDG PET/CT parameters and correlations with tumor-absorbed doses in a phase 1 trial of 177 Lu-lilotomab satetraxetan for treatment of relapsed non-Hodgkin lymphoma, PMID: 33196921
Biodistribution and Dosimetry Results from a Phase 1 Trial of Therapy with the Antibody-Radionuclide Conjugate 177 Lu-Lilotomab Satetraxetan, PMID: 28848035
Red Marrow-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with 177Lu-Lilotomab Satetraxetan, a Novel Anti-CD37 Antibody-Radionuclide Conjugate, PMID: 27587710
Tumor-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with the Anti-CD37 Antibody Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan, PMID: 27493270
The Dual Cell Cycle Kinase Inhibitor JNJ-7706621 Reverses Resistance to CD37-Targeted Radioimmunotherapy in Activated B Cell Like Diffuse Large B Cell Lymphoma Cell Lines, PMID: 31850205
Myelosuppression in patients treated with 177 Lutetium-lilotomab satetraxetan can be predicted with absorbed dose to the red marrow as the only variable, PMID: 34425735
Targeted alpha therapy for chronic lymphocytic leukaemia and non-Hodgkin's lymphoma with the anti-CD37 radioimmunoconjugate 212Pb-NNV003, PMID: 32187209
FDG PET/CT and Dosimetric Studies of 177Lu-Lilotomab Satetraxetan in a First-in-Human Trial for Relapsed Indolent non-Hodgkin Lymphoma-Are We Hitting the Target?, PMID:35486292
Myelosuppression in patients treated with 177Lutetium-lilotomab satetraxetan can be predicted with absorbed dose to the red marrow as the only variable., PMID:34425735
FDG PET/CT parameters and correlations with tumor-absorbed doses in a phase 1 trial of 177Lu-lilotomab satetraxetan for treatment of relapsed non-Hodgkin lymphoma., PMID:33196921
Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma., PMID:32877524
177Lu-Lilotomab Satetraxetan Has the Potential to Counteract Resistance to Rituximab in Non-Hodgkin Lymphoma., PMID:32245896
Targeted alpha therapy for chronic lymphocytic leukaemia and non-Hodgkin's lymphoma with the anti-CD37 radioimmunoconjugate 212Pb-NNV003., PMID:32187209
The Dual Cell Cycle Kinase Inhibitor JNJ-7706621 Reverses Resistance to CD37-Targeted Radioimmunotherapy in Activated B Cell Like Diffuse Large B Cell Lymphoma Cell Lines., PMID:31850205
The therapeutic effectiveness of 177Lu-lilotomab in B-cell non-Hodgkin lymphoma involves modulation of G2/M cell cycle arrest., PMID:31836849
Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine., PMID:31481583
Combination of 177 Lu-lilotomab with rituximab significantly improves the therapeutic outcome in preclinical models of non-Hodgkin's lymphoma., PMID:29993152
Pre-dosing with lilotomab prior to therapy with 177Lu-lilotomab satetraxetan significantly increases the ratio of tumor to red marrow absorbed dose in non-Hodgkin lymphoma patients., PMID:29470615
Biodistribution and Dosimetry Results from a Phase 1 Trial of Therapy with the Antibody-Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan., PMID:28848035
Red Marrow-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with 177Lu-Lilotomab Satetraxetan, a Novel Anti-CD37 Antibody-Radionuclide Conjugate., PMID:27587710
Tumor-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with the Anti-CD37 Antibody Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan., PMID:27493270