Catalog No.
PHD35801
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human CEL (Asp117-Glu361).
Tested applications
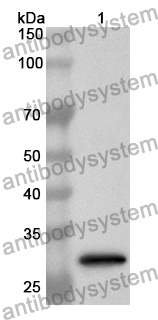
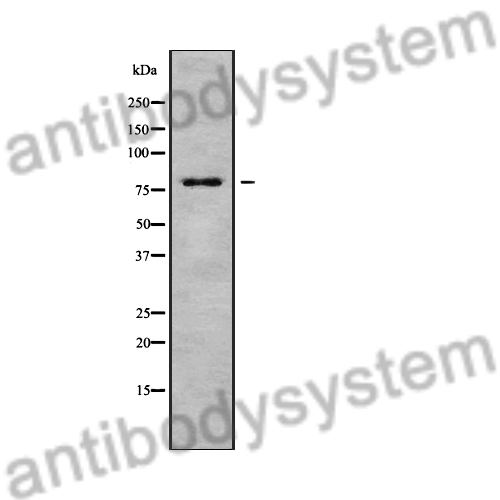
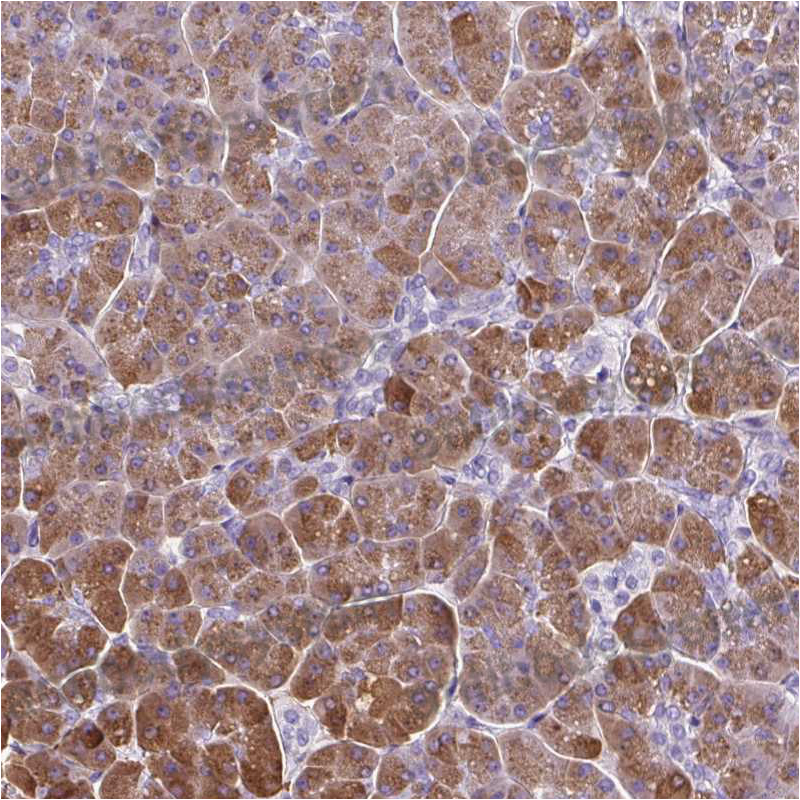
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
Carboxyl ester lipase, Bucelipase, Sterol esterase, Bile salt-activated lipase, BAL, Bile salt-stimulated lipase, Cholesterol esterase, Pancreatic lysophospholipase, CEL, BSSL
Purification
Purified by antigen affinity column.
Accession
P19835
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Cost effectiveness of mosunetuzumab and CAR-T cell therapy in relapsed/refractory follicular lymphoma., PMID:40488632
FDA Approval Summary: Idecabtagene Vicleucel for the Treatment of Triple-Class Exposed, Relapsed or Refractory Multiple Myeloma., PMID:40465403
Health and Economic Impact of Vein-to-Vein Time in CAR T-Cell Therapy in the Second-Line Treatment of Relapsed/Refractory Large B-Cell Lymphoma: A US Cost-Effectiveness Analysis., PMID:40373976
Genotype, Phenotype, and Clinical Characteristics of Maturity-onset Diabetes of the Young (MODY): Predominance of GCK-MODY., PMID:40338033
Intravenous Immunoglobulin (IVIG) for Patients with Severe Neurotoxicity Associated with Chimeric Antigen Receptor T-Cell (CAR-T) Therapy., PMID:40332772
Long-Term Effects of Atidarsagene Autotemcel for Metachromatic Leukodystrophy., PMID:40267426
Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: 5-year follow-up from the randomized phase III KEYNOTE-048 study., PMID:40262400
Case Report: Successful use of emapalumab in adult B-cell acute lymphoblastic leukemia experiencing severe neurotoxicity and hemophagocytic lymphohistiocytosis-like features after CAR-T cell therapy., PMID:40255392
Budget impact of introducing glofitamab for treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy in the United States., PMID:40163049
Impact of Different Definitions of Vaso-Occlusion on Efficacy Assessments in Sickle Cell Disease Clinical Trials., PMID:40146367
Current Treatment Strategies for Multiple Myeloma at First Relapse., PMID:40095642
Injectable celastrol-loading emulsion hydrogel for immunotherapy of low-immunogenic cancer., PMID:40050985
Matching-adjusted indirect comparison of efficacy and safety of lisocabtagene maraleucel and mosunetuzumab for the treatment of third-line or later relapsed or refractory follicular lymphoma., PMID:40045329
Celastrol-loaded ginsenoside Rg3 liposomes enhance anti-programmed death ligand 1 immunotherapy by inducing immunogenic cell death in triple-negative breast cancer., PMID:39986227
Always more immunotherapy in the management of B-lineage acute lymphoblastic leukemia., PMID:39954668
Allogeneic Chimeric Antigen Receptor T-Cell Products Cemacabtagene Ansegedleucel/ALLO-501 in Relapsed/Refractory Large B-Cell Lymphoma: Phase I Experience From the ALPHA2/ALPHA Clinical Studies., PMID:39946666
Improving Treatment Options for Patients with Double Refractory CLL., PMID:39941798
[The first Italian patient affected by diffuse large B-cell lymphoma treated with axi-cel in the second line: innovation and tradition]., PMID:39931889
First seroprevalence study of West Nile Virus (WNV) infection in blood donors after the upsurge of West Nile Neuroinvasive Disease (WNND) cases in southern Italy in 2023., PMID:39930346
Population Pharmacokinetics of Orvacabtagene Autoleucel, an Autologous BCMA-Directed Chimeric Antigen Receptor T-cell Product, in Patients with Relapsed/Refractory Multiple Myeloma., PMID:39836430
Idecabtagene vicleucel or ciltacabtagene autoleucel for relapsed or refractory multiple myeloma: An international multicenter study., PMID:39822585
Neoadjuvant or concurrent atezolizumab with chemoradiation for locally advanced cervical cancer: a randomized phase I trial., PMID:39788967
Updates on Chimeric Antigen Receptor T-Cells in Large B-Cell Lymphoma., PMID:39767716
Patient-reported outcomes following ciltacabtagene autoleucel or standard of care in patients with lenalidomide-refractory multiple myeloma (CARTITUDE-4): results from a randomised, open-label, phase 3 trial., PMID:39756844
An mpox quadrivalent mRNA vaccine elicits sustained and protective immunity in mice against lethal vaccinia virus challenge., PMID:39745170
Preclinical development of three novel CARs targeting CD79b for the treatment of non-Hodgkin's lymphoma and characterization of the loss of the target antigen., PMID:39694704
Real-world practitioner perceptions of CARTITUDE-4 results for patients with previously treated multiple myeloma., PMID:39691251
Bispecific antibodies targeting BCMA or GPRC5D are highly effective in relapsed myeloma after CAR T-cell therapy., PMID:39632797
Gene Expression Profiling of the Peritumoral Immune Cell Infiltrate of Penile Squamous Cell Carcinomas., PMID:39596210
Safety and Tolerability of Letetresgene Autoleucel (GSK3377794): Pilot Studies in Patients with Advanced Non-Small Cell Lung Cancer., PMID:39576208
Bilayered skin equivalent mimicking psoriasis as predictive tool for preclinical treatment studies., PMID:39558145
Equecabtagene Autoleucel in Patients With Relapsed or Refractory Multiple Myeloma: The FUMANBA-1 Nonrandomized Clinical Trial., PMID:39509090
Comparative Analysis of Bispecific Antibodies and CAR T-Cell Therapy in Follicular Lymphoma., PMID:39462177
Mechanisms of Resistance to Rituximab Used for the Treatment of Autoimmune Blistering Diseases., PMID:39459523
Gene therapy in transfusion-dependent non-β0/β0 genotype β-thalassemia: first real-world experience of beti-cel., PMID:39418614
[Role of CAR-T in multiple myeloma and coordination between referring and treating centers]., PMID:39358259
CAR-T cell therapy in relapsed or refractory multiple myeloma and access in Turkey., PMID:39267974
Updates on CAR T cell therapy in multiple myeloma., PMID:39261906
Effect of Bimagrumab on body composition: a systematic review and meta-analysis., PMID:39251484
Cost-per-responder analysis of patients with lenalidomide-refractory multiple myeloma receiving ciltacabtagene autoleucel in CARTITUDE-4., PMID:39234256
Evaluation of Anti-CAR Linker mAbs for CAR T Monitoring after BiTEs/bsAbs and CAR T-Cell Pretreatment., PMID:39200107
Tocilizumab Prophylaxis Following Axicabtagene Ciloleucel in Relapsed or Refractory Large B-Cell Lymphoma., PMID:39187161
Outcome of patients with large B-cell lymphoma treated with tafasitamab plus lenalidomide either before or after CAR T-cell therapy., PMID:39163620
Efficacy of CARVYKTI in CARTITUDE-4 versus other conventional treatment regimens for lenalidomide-refractory multiple myeloma using inverse probability of treatment weighting., PMID:39162049
Safety and immunogenicity of a next-generation live-attenuated yellow fever vaccine produced in a Vero cell line in the USA: a phase 1 randomised, observer-blind, active-controlled, dose-ranging clinical trial., PMID:39153488
Exploring the Diagnostic Complexity of Diabetes Subtypes in Pediatric Obesity: A Case Report of an Adolescent With Prader-Willi Phenotype and Literature Review., PMID:39135667
Secondary failure of lentiviral vector gene therapy in a cerebral adrenoleukodystrophy patient with an ABCD1 whole-gene deletion., PMID:39108094
Fast and furious: Changing gears on the road to cure with chimeric antigen receptor T cells in multiple myeloma., PMID:39095225
Impact of prior inotuzumab ozogamicin treatment on brexucabtagene autoleucel outcomes in adults with B-cell ALL., PMID:39093952
Practice efficiency and total cost of care with bispecifics and CAR-T in relapsed/refractory diffuse large B-cell lymphoma: an institutional perspective., PMID:38913826