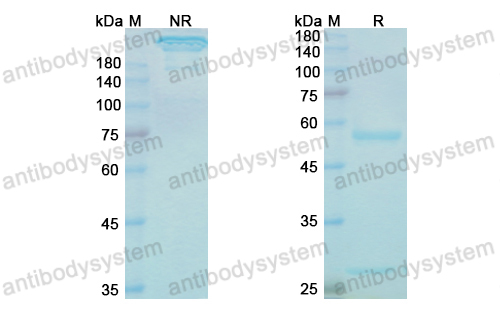
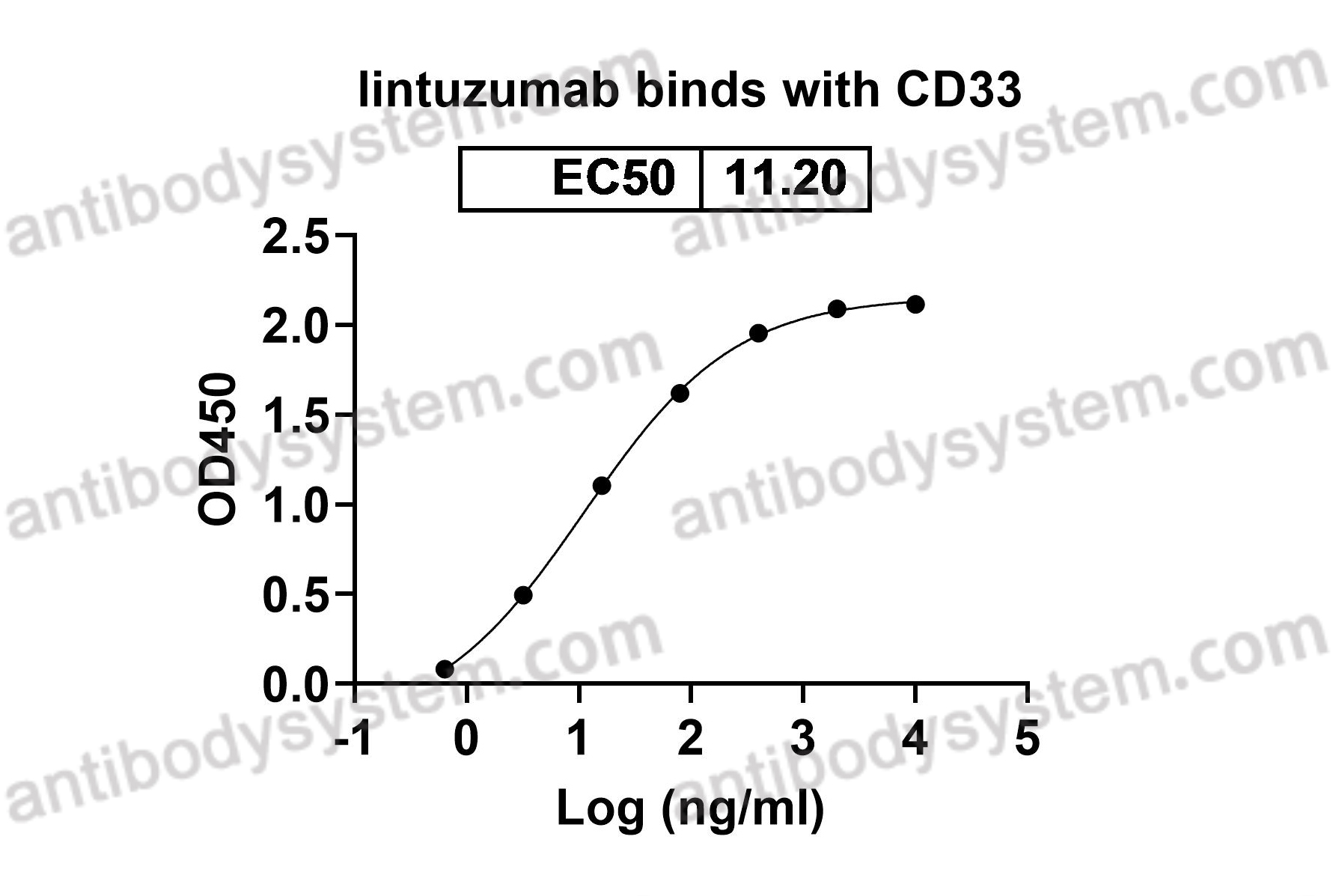
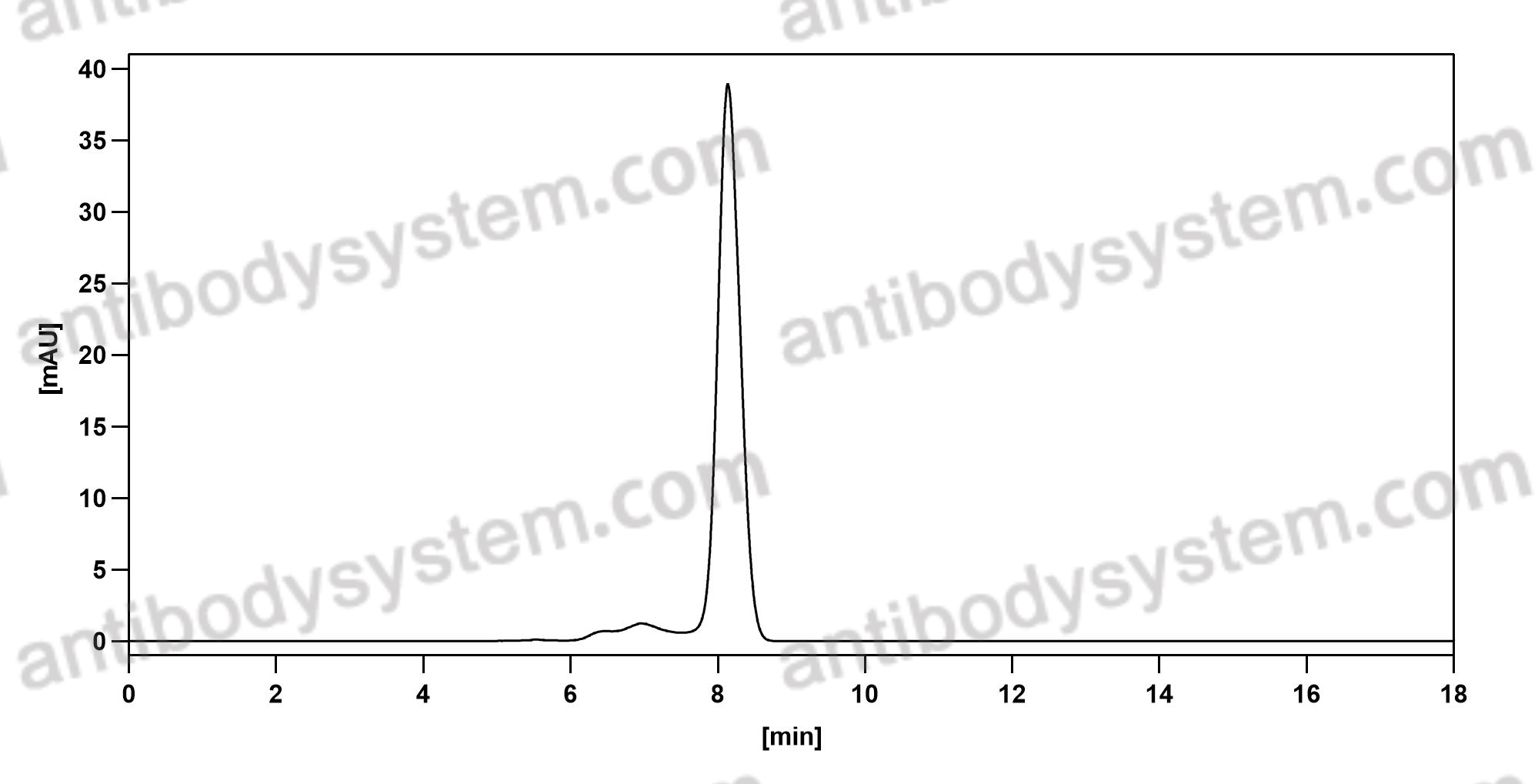
Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors, PMID: 29206680
Investigational CD33-targeted therapeutics for acute myeloid leukemia, PMID: 29534618
CD33-Targeted Therapies: Beating the Disease or Beaten to Death?, PMID: 32875599
Targeted Alpha-Particle Therapy for Hematologic Malignancies, PMID: 31253514
Targeted Alpha-Particle Therapy for Hematologic Malignancies, PMID: 32172800
Anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia, PMID: 20065652
Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies, PMID: 29793418
Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia, PMID: 20858843
5-azacytidine enhances the anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia, PMID: 20495353
225Ac-labeled CD33-targeting antibody reverses resistance to Bcl-2 inhibitor venetoclax in acute myeloid leukemia models, PMID: 33347715
Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia, PMID: 22801961
Targeted alpha-particle immunotherapy for acute myeloid leukemia, PMID: 24857092
Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia, PMID: 15961759
Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial, PMID: 19557623
What happened to anti-CD33 therapy for acute myeloid leukemia?, PMID: 22109628
Newer monoclonal antibodies for hematological malignancies, PMID: 18565392
Novel drugs for older patients with acute myeloid leukemia, PMID: 25142817
Harnessing the Immune System Against Leukemia: Monoclonal Antibodies and Checkpoint Strategies for AML, PMID: 28321813
New agents: great expectations not realized, PMID: 24309529
Ab therapy of AML: native anti-CD33 Ab and drug conjugates, PMID: 17917880
Advances in immunotherapy for pediatric acute myeloid leukemia, PMID: 28945115
Genetics of CD33 in Alzheimer's disease and acute myeloid leukemia, PMID: 25762156
Integration of monoclonal antibodies and immunoconjugates into the treatment of acute myeloid leukemia, PMID: 18300754
Antibody therapy for acute myeloid leukemia, PMID: 18381105
ADCs Show Promise in Leukemias, PMID: 27388473
Gateways to clinical trials, PMID: 20094643
Gateways to clinical trials, PMID: 16395422
Gateways to clinical trials, PMID: 12949633
Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands, PMID: 23014531
Precision 're'arming of CD33 antibodies, PMID: 23970353
CD33 splice site genotype was not associated with outcomes of patients receiving the anti-CD33 drug conjugate SGN-CD33A, PMID: 31439003
In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia, PMID: 27535972
Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor, PMID: 15661266
SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML, PMID: 23770776
Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates, PMID: 14734685
Phase III trial of a humanized anti-CD33 antibody (HuM195) in patients with relapsed or refractory acute myeloid leukemia, PMID: 12141950
Phase 1 study of lintuzumab-Ac225 combined with CLAG-M salvage therapy in relapsed/refractory acute myeloid leukemia., PMID:39955432
89Zr-immunoPET-guided selection of a CD33xIL15 fusion protein optimized for antitumor immune cell activation and in vivo tumour retention in acute myeloid leukaemia., PMID:38987489
Developing a membrane-proximal CD33-targeting CAR T cell., PMID:38772686
Targeting CD33+ Acute Myeloid Leukemia with GLK-33, a Lintuzumab-Auristatin Conjugate with a Wide Therapeutic Window., PMID:38561023
HuM195 and its single-chain variable fragment increase Aβ phagocytosis in microglia via elimination of CD33 inhibitory signaling., PMID:38383769
In Vitro and In Vivo Characterization of 89Zirconium-Labeled Lintuzumab Molecule., PMID:36235126
Where do we stand with radioimmunotherapy for acute myeloid leukemia?, PMID:35350938
Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab., PMID:35247915
Radiation Safety Considerations and Clinical Advantages of α-Emitting Therapy Radionuclides., PMID:34750237
Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design., PMID:34531250
225Ac-labeled CD33-targeting antibody reverses resistance to Bcl-2 inhibitor venetoclax in acute myeloid leukemia models., PMID:33347715
CD33-Targeted Therapies: Beating the Disease or Beaten to Death?, PMID:32875599
Targeted Alpha-Particle Therapy for Hematologic Malignancies., PMID:32172800
CD33 splice site genotype was not associated with outcomes of patients receiving the anti-CD33 drug conjugate SGN-CD33A., PMID:31439003
Targeted Alpha-Particle Therapy for Hematologic Malignancies., PMID:31253514
Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies., PMID:29793418
Investigational CD33-targeted therapeutics for acute myeloid leukemia., PMID:29534618
Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors., PMID:29206680
Advances in immunotherapy for pediatric acute myeloid leukemia., PMID:28945115
Harnessing the Immune System Against Leukemia: Monoclonal Antibodies and Checkpoint Strategies for AML., PMID:28321813
In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia., PMID:27535972
ADCs Show Promise in Leukemias., PMID:27388473
Genetics of CD33 in Alzheimer's disease and acute myeloid leukemia., PMID:25762156
Novel drugs for older patients with acute myeloid leukemia., PMID:25142817
Targeted alpha-particle immunotherapy for acute myeloid leukemia., PMID:24857092
New agents: great expectations not realized., PMID:24309529
Precision 're'arming of CD33 antibodies., PMID:23970353
SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML., PMID:23770776
Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands., PMID:23014531
Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia., PMID:22801961
What happened to anti-CD33 therapy for acute myeloid leukemia?, PMID:22109628
Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia., PMID:20858843
5-azacytidine enhances the anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia., PMID:20495353
Gateways to clinical trials., PMID:20094643
Anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia., PMID:20065652
Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial., PMID:19557623
Newer monoclonal antibodies for hematological malignancies., PMID:18565392
Antibody therapy for acute myeloid leukemia., PMID:18381105
Integration of monoclonal antibodies and immunoconjugates into the treatment of acute myeloid leukemia., PMID:18300754
Ab therapy of AML: native anti-CD33 Ab and drug conjugates., PMID:17917880
Gateways to clinical trials., PMID:16395422
Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia., PMID:15961759
Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor., PMID:15661266
Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates., PMID:14734685
Gateways to clinical trials., PMID:12949633
Phase III trial of a humanized anti-CD33 antibody (HuM195) in patients with relapsed or refractory acute myeloid leukemia., PMID:12141950