Catalog No.
PHE11401
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human IDUA (Ala28-Trp306).
Tested applications
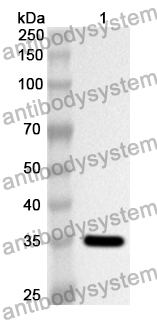
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
IDUA, Alpha-L-iduronidase
Purification
Purified by antigen affinity column.
Accession
P35475
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Antibodies to recombinant human alpha-L-iduronidase prevent disease correction in cortical bone in MPS I mice., PMID:40123743
α-L-iduronidase fused with humanized anti-human transferrin receptor antibody (lepunafusp alfa) for mucopolysaccharidosis type I: A phase 1/2 trial., PMID:38204164
Generation and Characterization of Iduronidase-Cleavable ADCs., PMID:38054705
Enzyme replacement with transferrin receptor-targeted α-L-iduronidase rescues brain pathology in mucopolysaccharidosis I mice., PMID:37251981
Ocular Tolerability and Immune Response to Corneal Intrastromal AAV-IDUA Gene Therapy in New Zealand White Rabbits., PMID:32542182
Neonatal combination therapy improves some of the clinical manifestations in the Mucopolysaccharidosis type I murine model., PMID:32439268
Intrastromal Gene Therapy Prevents and Reverses Advanced Corneal Clouding in a Canine Model of Mucopolysaccharidosis I., PMID:32330426
Dermatan sulfate epimerase 1 expression and mislocalization may interfere with dermatan sulfate synthesis and breast cancer cell growth., PMID:31972438
Targeting a Pre-existing Anti-transgene T Cell Response for Effective Gene Therapy of MPS-I in the Mouse Model of the Disease., PMID:31060789
Safe and Sustained Expression of Human Iduronidase After Intrathecal Administration of Adeno-Associated Virus Serotype 9 in Infant Rhesus Monkeys., PMID:31017018
Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1-2 trial., PMID:29976218
Plasma Pharmacokinetics of Valanafusp Alpha, a Human Insulin Receptor Antibody-Iduronidase Fusion Protein, in Patients with Mucopolysaccharidosis Type I., PMID:29442294
RTB lectin-mediated delivery of lysosomal α-l-iduronidase mitigates disease manifestations systemically including the central nervous system., PMID:29198892
Brain and Organ Uptake in the Rhesus Monkey in Vivo of Recombinant Iduronidase Compared to an Insulin Receptor Antibody-Iduronidase Fusion Protein., PMID:28279069
Neonatal Systemic AAV Induces Tolerance to CNS Gene Therapy in MPS I Dogs and Nonhuman Primates., PMID:26022732
Effects of enzyme replacement therapy started late in a murine model of mucopolysaccharidosis type I., PMID:25646802
α- L-iduronidase gene-based therapy using the phiC31 system to treat mucopolysaccharidose type I mice., PMID:25597593
Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I., PMID:25027660
Mesenchymal stem cells do not prevent antibody responses against human α-L-iduronidase when used to treat mucopolysaccharidosis type I., PMID:24642723
Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice., PMID:23562162
IgG-enzyme fusion protein: pharmacokinetics and anti-drug antibody response in rhesus monkeys., PMID:23249376
Glycemic control and chronic dosing of rhesus monkeys with a fusion protein of iduronidase and a monoclonal antibody against the human insulin receptor., PMID:22822036
Intraperitoneal implant of recombinant encapsulated cells overexpressing alpha-L-iduronidase partially corrects visceral pathology in mucopolysaccharidosis type I mice., PMID:22472038
Reversal of lysosomal storage in brain of adult MPS-I mice with intravenous Trojan horse-iduronidase fusion protein., PMID:21667973
Evaluation of a general practice based hepatitis C virus screening intervention., PMID:19728405
A new generation of neurobiological drugs engineered to overcome the challenges of brain drug delivery., PMID:19180267
Cholera toxin subunit B detection in microfluidic devices., PMID:18777170
Targeting of the CNS in MPS-IH using a nonviral transferrin-alpha-L-iduronidase fusion gene product., PMID:18523448
Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome., PMID:18037941
Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier., PMID:17680664
Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation., PMID:17311010
Synthesis of enzymatically active human alpha-L-iduronidase in Arabidopsis cgl (complex glycan-deficient) seeds., PMID:17177794
Limited transgene immune response and long-term expression of human alpha-L-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy., PMID:17044753
Gene therapy of the brain in the dog model of Hurler's syndrome., PMID:16718701
Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig., PMID:16698321
Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice., PMID:16482204
Enzyme replacement therapy in mucopolysaccharidosis type I., PMID:15895714
Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector., PMID:15703491
Chromosomal localization of the human alpha-L-iduronidase gene (IDUA) to 4p16.3., PMID:2220820