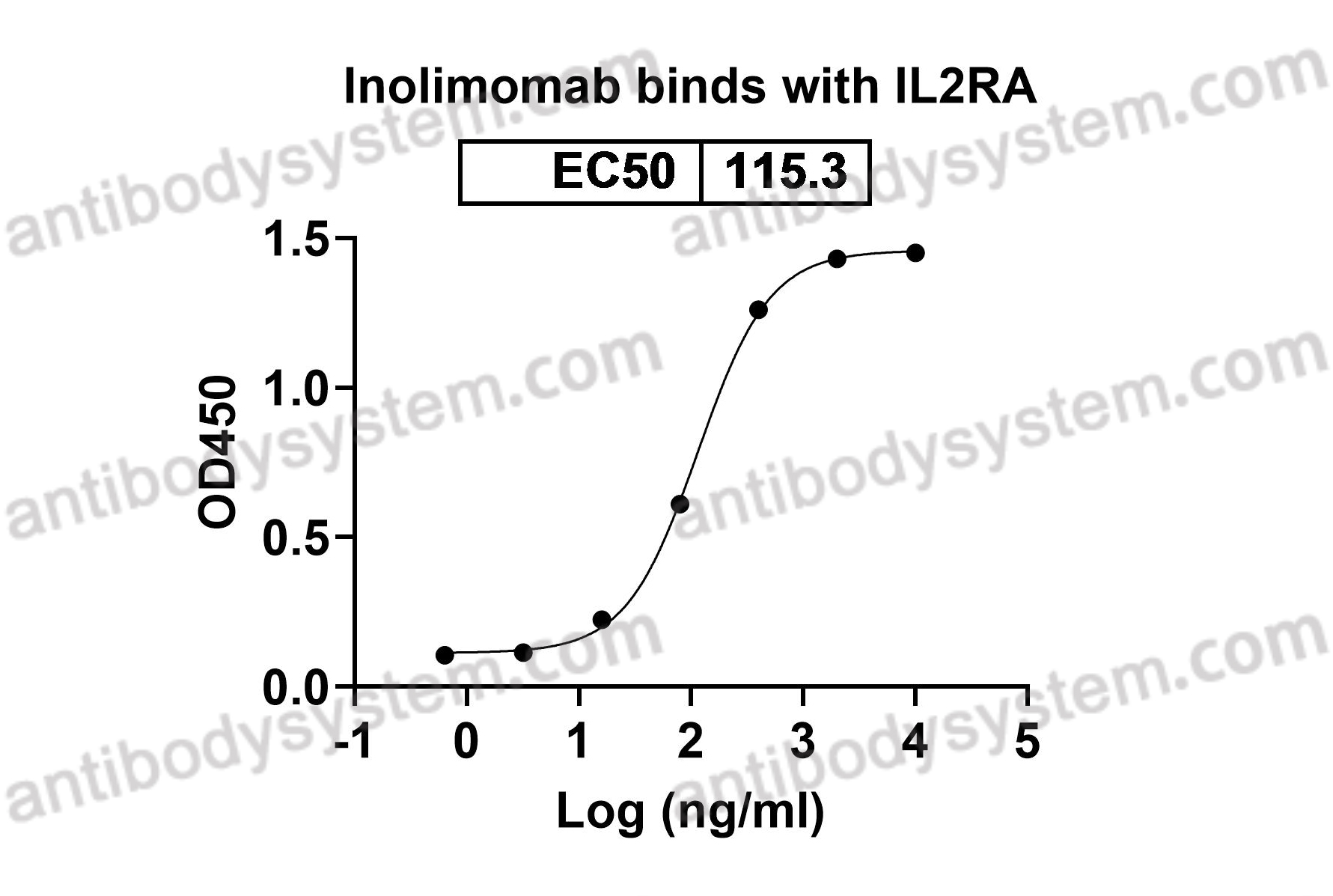
Inolimomab (OPi), PMID: 12431019
A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD, PMID: 27899357
Inolimomab in steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: retrospective analysis and comparison with other interleukin-2 receptor antibodies, PMID: 16210965
Long-term follow-up of a phase 3 clinical trial of inolimomab for the treatment of primary steroid refractory aGVHD, PMID: 30670534
Combination Therapy with Inolimomab and Etanercept for Severe Steroid-Refractory Acute Graft-versus-Host Disease, PMID: 26386320
Exposure-effect population model of inolimomab, a monoclonal antibody administered in first-line treatment for acute graft-versus-host disease, PMID: 17465640
Updated experience with inolimomab as treatment for corticosteroid-refractory acute graft-versus-host disease, PMID: 23178634
Inolimomab. Anti-CD25 monoclonal antibody B-B10, anti-interleukin-2 receptor monoclonal antibody B-B10, B-B10, BT 563, Leukotac, PMID: 10565995
Encouraging results with inolimomab (anti-IL-2 receptor) as treatment for refractory acute graft-versus-host disease, PMID: 17085306
Long-term follow-up of corticosteroid refractory acute GVHD treated with an Inolimomab-based algorithm: a single center experience, PMID: 23503532
Immunotherapies in dermatologic disorders, PMID: 22703856
Adverse events in second- and third-line treatments for acute and chronic graft- versus-host disease: systematic review, PMID: 33343855
Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment, PMID: 21736868
The use of monoclonal antibodies in immune-mediated hematologic disorders, PMID: 22703857
Gateways to clinical trials, PMID: 16894408
Gateways to clinical trials, PMID: 16801985
Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease, PMID: 27595281
[Role of monoclonal antibodies in the treatment of acute graft versus host disease], PMID: 17237003
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies, PMID: 33807678
Immunosuppressive T-cell antibody induction for heart transplant recipients, PMID: 24297433
Interleukin-2 receptor antibody therapy, PMID: 9404968
MEK-dependent IL-8 induction regulates the invasiveness of triple-negative breast cancer cells, PMID: 26537583
IL-7 and IL-15 bypass the immunosuppressive action of anti-CD25 monoclonal antibodies, PMID: 11377516
Ten-year follow-up of a prospective, randomized trial of BT563/bb10 versus anti-thymocyte globulin as induction therapy after heart transplantation, PMID: 16962480
Long-time survival after unrelated bone marrow transplantation in children and adolescents and targeted therapy with CD25 blockade to prevent GVHD, PMID: 15175962
In vitro and in vivo effects of BT 563, an anti-interleukin-2 receptor monoclonal antibody, PMID: 11271306
IL-2 receptor antibody induction increases the risk for chronic rejection after liver transplantation, PMID: 11267360
Anti-IL-2 receptor BT563 versus placebo: a randomized trial for induction therapy after liver transplantation, PMID: 9723425
Treatment of steroid-resistant acute graft-vs-host disease after allogeneic marrow transplantation with anti-interleukin-2 receptor antibody (BT563), PMID: 7998086
Outcome following orthotopic liver transplantation in HBsAg-positive patients using short- or long-term immunoprophylaxis, PMID: 7527983
Ten-year follow-up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin-2 receptor, PMID: 15859929
Rejection prophylaxis with interleukin-2 receptor antibody BT 563: mechanisms of action on human cells, PMID: 14621910
Spontaneous proliferation of peripheral blood lymphocytes as an indicator of intragraft immune activation in liver transplant patients, PMID: 7949544
A randomized, placebo-controlled trial with anti-interleukin-2 receptor antibody for immunosuppressive induction therapy after liver transplantation, PMID: 9686324
Blockade of the interleukin (IL)-2/IL-2 receptor pathway with a monoclonal anti-IL-2 receptor antibody (BT563) does not prevent the development of acute heart allograft rejection in humans, PMID: 9484761
Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter phase III study, PMID: 7491697
A prospective randomized trial comparing interleukin-2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation, PMID: 9210503
De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or antithymocyte globulin, PMID: 9305716
[Salvage therapy with anti-IL-2 receptor antibodies in high-risk kidney transplant patients], PMID: 9765770
A randomized trial comparing anti-interleukin-2 receptor antibody and placebo for immunosuppressive therapy after OLT, PMID: 9636586
Comparison of quadruple induction including ATG or IL-2R antibody with FK506-based therapy after liver transplantation, PMID: 9636583
Quadruple induction therapy including antithymocyte globulin or interleukin-2 receptor antibody or FK 506-based induction therapy after liver transplantation, PMID: 10083151
Common and sequential overexpression patterns of T-helper cytokines during acute (cellular) rejection, and correlation of proinflammatory cytokine expression with chronic (ductopenic) rejection of human liver allografts: a study under cyclosporine, FK 506, and quadruple BT 563 immunosuppression, PMID: 7533370
Peripheral blood monitoring during and after rejection-prophylaxis with a monoclonal anti-interleukin-2-receptor antibody in kidney and heart transplant recipients, PMID: 7879206
Interleukin-2 receptor antibody versus antithymocyte globulin as part of quadruple induction therapy after orthotopic liver transplantation: a randomized study, PMID: 8962241
Interleukin-2 receptor antibody vs ATG for induction immunosuppression after liver transplantation: initial results of a prospective randomized trial, PMID: 7878826
Antithymocyte globulin or interleukin 2 receptor antibody for immunosuppressive induction therapy after orthotopic liver transplantation: a follow-up study, PMID: 8356721
BT563 significantly decreases sIL-2R plasma levels despite acute allograft rejection, PMID: 8962203
Quadruple immunosuppression including a new IL-2-receptor antibody and the incidence of infections after liver transplantation, PMID: 14621765
Cytokine secretion capacity of mononuclear cells in renal transplant recipients: the effect of two different dose schedules, PMID: 8356715
Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials., PMID:37409129
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease., PMID:34621279
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies., PMID:33807678
Adverse events in second- and third-line treatments for acute and chronic graft-versus-host disease: systematic review., PMID:33343855
Long-term follow-up of a phase 3 clinical trial of inolimomab for the treatment of primary steroid refractory aGVHD., PMID:30670534
A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD., PMID:27899357
Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease., PMID:27595281
MEK-dependent IL-8 induction regulates the invasiveness of triple-negative breast cancer cells., PMID:26537583
Combination Therapy with Inolimomab and Etanercept for Severe Steroid-Refractory Acute Graft-versus-Host Disease., PMID:26386320
Immunosuppressive T-cell antibody induction for heart transplant recipients., PMID:24297433
Long-term follow-up of corticosteroid refractory acute GVHD treated with an Inolimomab-based algorithm: a single center experience., PMID:23503532
Updated experience with inolimomab as treatment for corticosteroid-refractory acute graft-versus-host disease., PMID:23178634
The use of monoclonal antibodies in immune-mediated hematologic disorders., PMID:22703857
Immunotherapies in dermatologic disorders., PMID:22703856
Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment., PMID:21736868
Exposure-effect population model of inolimomab, a monoclonal antibody administered in first-line treatment for acute graft-versus-host disease., PMID:17465640
[Role of monoclonal antibodies in the treatment of acute graft versus host disease]., PMID:17237003
Encouraging results with inolimomab (anti-IL-2 receptor) as treatment for refractory acute graft-versus-host disease., PMID:17085306
Ten-year follow-up of a prospective, randomized trial of BT563/bb10 versus anti-thymocyte globulin as induction therapy after heart transplantation., PMID:16962480
Gateways to clinical trials., PMID:16894408
Gateways to clinical trials., PMID:16801985
Inolimomab in steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: retrospective analysis and comparison with other interleukin-2 receptor antibodies., PMID:16210965
Ten-year follow-up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin-2 receptor., PMID:15859929
Long-time survival after unrelated bone marrow transplantation in children and adolescents and targeted therapy with CD25 blockade to prevent GVHD., PMID:15175962
Inolimomab (OPi)., PMID:12431019
Chronic rejection after orthotopic liver transplantation is increased under induction therapy with interleukin-2 receptor antibody BT563., PMID:11377531
IL-7 and IL-15 bypass the immunosuppressive action of anti-CD25 monoclonal antibodies., PMID:11377516
IL-2 receptor antibody induction increases the risk for chronic rejection after liver transplantation., PMID:11267360
Impact of primary immunosuppression on the incidence of infectious complications after orthotopic liver transplantation., PMID:11120145
Inolimomab. Anti-CD25 monoclonal antibody B-B10, anti-interleukin-2 receptor monoclonal antibody B-B10, B-B10, BT 563, Leukotac., PMID:10565995
Quadruple induction therapy including antithymocyte globulin or interleukin-2 receptor antibody or FK 506-based induction therapy after liver transplantation., PMID:10083151
Indication for mycophenolate mofetil therapy in hepatitis C patients undergoing liver transplantation., PMID:9723453
Anti-IL-2 receptor BT563 versus placebo: a randomized trial for induction therapy after liver transplantation., PMID:9723425
A randomized, placebo-controlled trial with anti-interleukin-2 receptor antibody for immunosuppressive induction therapy after liver transplantation., PMID:9686324
Blockade of acute grade IV skin and eye graft-versus-host disease by anti-interleukin-2 receptor monoclonal antibody in genoidentical bone marrow transplantation setting., PMID:9677728
Immunosuppression and incidence of opportunistic pneumonias after liver transplantation., PMID:9636599
Indications for mycophenolate mofetil therapy in hepatitis C-patients undergoing liver transplantation., PMID:9636596
A randomized trial comparing anti-interleukin-2 receptor antibody and placebo for immunosuppressive therapy after OLT., PMID:9636586
Comparison of quadruple induction including ATG or IL-2R antibody with FK506-based therapy after liver transplantation., PMID:9636583
Blockade of the interleukin (IL)-2/IL-2 receptor pathway with a monoclonal anti-IL-2 receptor antibody (BT563) does not prevent the development of acute heart allograft rejection in humans., PMID:9484761
Interleukin-2 receptor antibody therapy., PMID:9404968
De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or antithymocyte globulin., PMID:9305716
A prospective randomized trial comparing interleukin-2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation., PMID:9210503
Rescue therapy with interleukin-2 receptor antibody in high risk kidney transplant patients: a 3-year follow-up study., PMID:9123020
[Salvage therapy with anti-IL-2 receptor antibodies in high-risk kidney transplant patients]., PMID:9765770
Long-term follow-up after induction treatment with monoclonal anti-interleukin-2 receptor antibody (BT563) in kidney allograft recipients: a double-blind, placebo-controlled trial., PMID:8962247
Influence of the anti-CD25 monoclonal antibody BT563 on clinical and biological rejection after orthotopic liver transplantation., PMID:8962244
Interleukin-2 receptor antibody versus antithymocyte globulin as part of quadruple induction therapy after orthotopic liver transplantation: a randomized study., PMID:8962241
BT563 significantly decreases sIL-2R plasma levels despite acute allograft rejection., PMID:8962203
The release of cytokines, adhesion molecules, and extracellular matrix parameters during and after reperfusion in human liver transplantation., PMID:8900313