Catalog No.
PHD50901
Species reactivity
Human, Mouse, Rat
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human IDS (Arg95-Pro289).
Tested applications
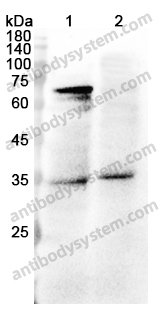
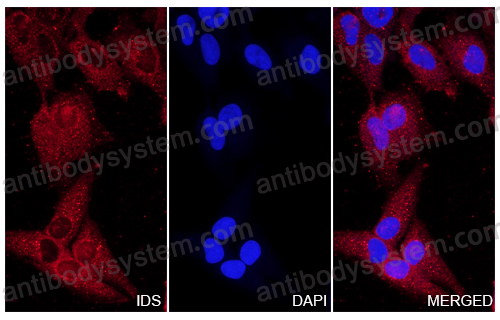
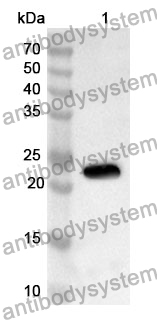
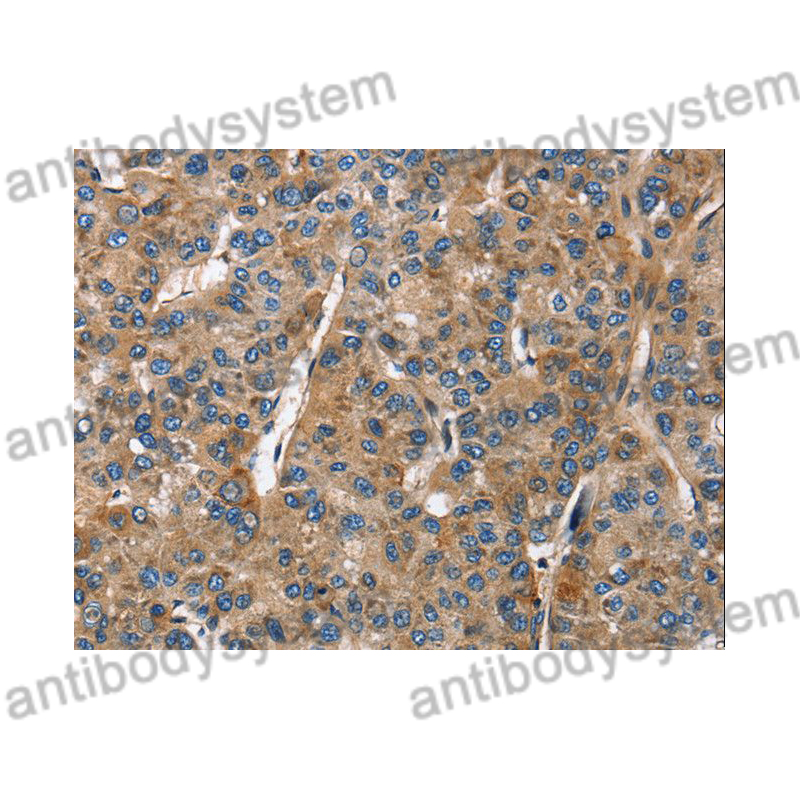
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
Alpha-L-iduronate sulfate sulfatase,Iduronate 2-sulfatase,SIDS,Idursulfase,IDS
Purification
Purified by antigen affinity column.
Accession
P22304
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Parameter-Optimized Fabrication of Submicron Nanoelectrospray Emitters for Enhanced Native Mass Spectrometry., PMID:40456619
Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies., PMID:40004506
Anti-Idiotypic Antibody as a Booster Vaccine Against Respiratory Syncytial Virus., PMID:39852814
In vivo brain delivery of BBB-enabled iduronate 2-sulfatase in rats., PMID:39810248
Construction of in situ modulated controlled growth of MOF-on-mof impedimetric assembly for the practical minimal level assessment of anti-mullerian hormone., PMID:39754847
Trajectory of beta cell function and insulin clearance in stage 2 type 1 diabetes: natural history and response to teplizumab., PMID:39560746
Ultrasensitive aflatoxin B1 detection based on vertical organic electrochemical transistor., PMID:39423541
A Programme of Hepatitis C Surveillance With Active Linkage to Care (HEAL) for Inpatients in Two Tertiary Hospitals in Jiangsu, China., PMID:39382123
Prognostic analysis of peritoneal washing cytology during interval debulking surgery in advanced ovarian cancer., PMID:39182152
In Vivo Proximity Cross-Linking and Immunoprecipitation of Cell Wall Epitopes Identify Proteins Associated with the Biosynthesis of Matrix Polysaccharides., PMID:39072051
Novel approaches to enable equitable access to monoclonal antibodies in low- and middle-income countries., PMID:38950021
Phase I and Randomized Phase II Study of Ruxolitinib With Frontline Neoadjuvant Therapy in Advanced Ovarian Cancer: An NRG Oncology Group Study., PMID:38776484
Emerging treatments in HER2-positive advanced breast cancer: Keep raising the bar., PMID:38759648
Paracrinal regulation of neutrophil functions by coronaviral infection in iPSC-derived alveolar type II epithelial cells., PMID:38754785
Selection of positive controls and their impact on anti-drug antibody assay performance., PMID:38479453
Clinical characteristics and treatment modalities in women with newly diagnosed advanced high-grade serous epithelial ovarian cancer in Taiwan., PMID:38453530
Analytical and diagnostic performance of Theradiag i-Tracker assays on IDS-iSYS for infliximab and adalimumab therapeutic drug monitoring., PMID:38379379
Screening Non-neutralizing Anti-idiotype Antibodies Against a Drug Candidate for Total Pharmacokinetic and Target Engagement Assay., PMID:38267774
A close-up view of the Hunter syndrome., PMID:38241811
Automated Bioanalytical Workflow for Ligand Binding-Based Pharmacokinetic Assay Development., PMID:38156369
Associations of Changes in Bone Turnover Markers with Change in Bone Mineral Density in Kidney Transplant Patients., PMID:38030558
The impact of erythropoiesis-stimulating agents administration concomitantly with adjuvant anti-HER2 treatments on the outcomes of patients with early breast cancer: a sub-analysis of the ALTTO study., PMID:37938495
Trends in prevalence, treatment use, and disease burden in patients with eosinophilic granulomatosis with polyangiitis in Japan: Real-world database analysis., PMID:37930840
Immunological and clinicopathological features predict HER2-positive breast cancer prognosis in the neoadjuvant NeoALTTO and CALGB 40601 randomized trials., PMID:37923752
Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:37919269
Transferrin Receptor-Targeted Iduronate-2-sulfatase Penetrates the Blood-Retinal Barrier and Improves Retinopathy in Mucopolysaccharidosis II Mice., PMID:37860991
Real-world clinical outcomes of patients with stage I HER2-positive breast cancer treated with adjuvant paclitaxel and trastuzumab., PMID:37562696
Modeling the kinetics of the neutralizing antibody response against SARS-CoV-2 variants after several administrations of Bnt162b2., PMID:37549192
Phage-derived anti-idiotype and anti-YTE antibodies in development of MK-1654 pharmacokinetic and immune response assays., PMID:37515532
Ten-year safety and clinical benefit from open-label etanercept treatment in children and young adults with juvenile idiopathic arthritis., PMID:37140539
Real-world study of bevacizumab treatment in patients with ovarian cancer: a Chinese single-institution study of 155 patients., PMID:37055754
Immunoinformatics Study: Multi-Epitope Based Vaccine Design from SARS-CoV-2 Spike Glycoprotein., PMID:36851275
Immunotherapy in breast cancer: an overview of current strategies and perspectives., PMID:36781869
SpikeScape: A Tool for Analyzing Structural Diversity in Experimental Structures of the SARS-CoV-2 Spike Glycoprotein., PMID:36758040
The Heterogeneity of Ovomucoid-Specific IgE Idiotype Is Associated With Egg Allergy Symptom Severity., PMID:36693362
Cardiac safety of dual anti-HER2 blockade with pertuzumab plus trastuzumab in early HER2-positive breast cancer in the APHINITY trial., PMID:36681013
Use of durvalumab in stage III non-small-cell lung cancer based on eligibility for the PACIFIC study., PMID:36627112
A New Laboratory Workflow Integrating the Free Light Chains Kappa Quotient into Routine CSF Analysis., PMID:36421703
Outcomes of patients with small and node-negative HER2-positive early breast cancer treated with adjuvant chemotherapy and anti-HER2 therapy-a sub-analysis of the ALTTO study., PMID:36050448
Immune response to anti-SARS-CoV-2 prime-vaccination in patients with cancer: a systematic review and meta-analysis., PMID:35867203
Selective quantification of the 22-kDa isoform of human growth hormone 1 in serum and plasma by immunocapture and LC-MS/MS., PMID:35838770
Real-life data on treatment and outcomes in advanced ovarian cancer: An observational, multinational cohort study (RESPONSE trial)., PMID:35714310
Standardisation of ACPA tests: evaluation of a new candidate reference preparation., PMID:35697487
Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis., PMID:35680967
Effect of Anti-Iduronate 2-Sulfatase Antibodies in Patients with Mucopolysaccharidosis Type II Treated with Enzyme Replacement Therapy., PMID:35568060
Exploration of quantitative site-specific serum O-glycoproteomics with isobaric labeling for the discovery of putative O-glycoprotein biomarkers., PMID:35507764
Screening and identification of vancomycin anti-idiotypic antibodies for against Staphylococcus aureus from a human phage display domain antibody library., PMID:35504507
Impact of an anti-infective screening and monitoring protocol together with infectious disease consultation in preventing infective adverse events in patients treated with anti-CD20/CD52 agents for multiple sclerosis., PMID:35487032
Six-year absolute invasive disease-free survival benefit of adding adjuvant pertuzumab to trastuzumab and chemotherapy for patients with early HER2-positive breast cancer: A Subpopulation Treatment Effect Pattern Plot (STEPP) analysis of the APHINITY (BIG 4-11) trial., PMID:35313167
Molecular architecture determines brain delivery of a transferrin receptor-targeted lysosomal enzyme., PMID:35226042