Catalog No.
DHC90716
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
B-lymphocyte surface antigen B1, Membrane-spanning 4-domains subfamily A member 1, MS4A1, Leukocyte surface antigen Leu-16, B-lymphocyte antigen CD20, Bp35, CD20, T3E, T-cell surface antigen T3/Leu-4 epsilon chain, CD3e, CD3E, T-cell surface glycoprotein CD3 epsilon chain
Concentration
4 mg/ml
Endotoxin level
Please contact with the lab for this information.
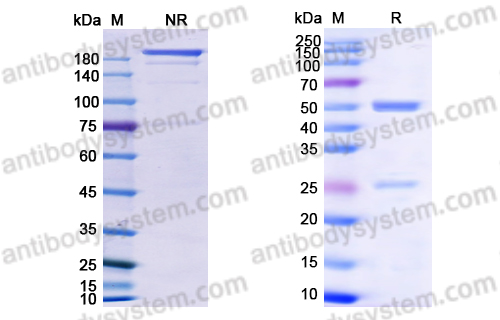
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P11836 & P07766
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Bispecific, BTCT4465A, RG-7828, RO7030816, CAS: 1905409-39-3
Clone ID
Mosunetuzumab
Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling, PMID: 32859946
Chemotherapy-Free Management of Follicular and Marginal Zone Lymphoma, PMID: 34017197
Bispecific antibodies for the treatment of lymphomas: Promises and challenges, PMID: 34105818
From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies., PMID:40508128
QSP modeling of loncastuximab tesirine with T-cell-dependent bispecific antibodies guides dose-regimen strategy., PMID:40500294
Cost effectiveness of mosunetuzumab and CAR-T cell therapy in relapsed/refractory follicular lymphoma., PMID:40488632
Indirect comparison of epcoritamab vs chemoimmunotherapy, mosunetuzumab, or odronextamab in follicular lymphoma., PMID:40472301
CD20×CD3 Bispecific Antibodies in B-NHL: A Review of Translational Science, Pharmacokinetics, Pharmacodynamics, and Dose Strategy in Clinical Research., PMID:40471801
Care Coordination for Mosunetuzumab Therapy in Patients With Follicular Lymphoma in Community Practices: Learnings From the MorningSun Study Investigators., PMID:40432369
Single-Agent and Associated Therapies with Monoclonal Antibodies: What About Follicular Lymphoma?, PMID:40427101
Recent development in bispecific antibody immunotherapy for hematological malignancies., PMID:40320222
Ethnic Sensitivity Assessment of Mosunetuzumab Pharmacokinetics and Pharmacodynamics in Chinese Patients With Relapsed or Refractory Follicular Lymphoma., PMID:40279333
SWOG 2308: Randomized Phase III Study of Mosunetuzumab Versus Rituximab for Low-Tumor Burden Follicular Lymphoma., PMID:40264918
A Systematic Literature Review of the Economic and Healthcare Resource Utilization Burden of Relapsed/Refractory Follicular Lymphoma., PMID:40263163
A Cost-Effectiveness Analysis of Mosunetuzumab vs Tisagenlecleucel for Treatment of Third- or Higher-Line (3L+) Relapsed or Refractory (R/R) Follicular Lymphoma (FL) in Italy., PMID:40256614
Matching-adjusted indirect comparison of efficacy and safety of lisocabtagene maraleucel and mosunetuzumab for the treatment of third-line or later relapsed or refractory follicular lymphoma., PMID:40045329
SOHO State of the Art Updates and Next Questions | Novel Immunotherapy Combinations for the Treatment of Indolent B-Cell Lymphoma., PMID:40011100
Antibody Therapy for Patients with Lymphoid Malignancies: Past and Present., PMID:40004173
The Development and Application of Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma., PMID:39997328
Comprehensive analysis of adverse events associated with T-cell engagers using the FAERS database., PMID:39982395
Mosunetuzumab next up to bat … is it a home run?, PMID:39946154
Two Are Better than One: The Bi-Specific Antibody Mosunetuzumab Reveals an Improved Immune Response of Vγ9Vδ2 T Cells Targeting CD20 in Malignant B Cells in Comparison to the Mono-Specific Antibody Obinutuzumab., PMID:39941030
New bispecific antibodies in diffuse large B-cell lymphoma., PMID:39911111
Mosunetuzumab plus Pola-CHP compared with Pola-R-CHP in previously untreated DLBCL: final results from a phase 2 study., PMID:39908481
Trials that treat what should be observed: SWOG 2308, a study of mosunetuzumab-axgb in asymptomatic low tumor burden follicular lymphoma., PMID:39894215
Matching plus regression adjustment for the estimation of the average treatment effect on survival outcomes: a case study with mosunetuzumab in relapsed/refractory follicular lymphoma., PMID:39893424
The emergence of bispecific T-cell engagers in the treatment of follicular and large B-cell lymphomas., PMID:39820375
Cytokine Dynamics in Action: A Mechanistic Approach to Assess Interleukin 6 Related Therapeutic Protein-Drug-Disease Interactions., PMID:39807804
Structural insight into CD20/CD3-bispecific antibodies by molecular modeling., PMID:39674067
A 1-year per-patient cost of therapy administration analysis of mosunetuzumab and tisagenlecleucel in relapsed or refractory follicular lymphoma patients receiving two or more lines of systemic therapy., PMID:39659736
Efficacy and safety of mosunetuzumab monotherapy for Japanese patients with relapsed/refractory follicular lymphoma: FLMOON-1., PMID:39652156
Sequencing bispecific antibodies and CAR T cells for FL., PMID:39643999
Bispecific antibodies for the treatment of hematologic malignancies: The magic is T-cell redirection., PMID:39617677
Impact of prior CAR T-cell therapy on mosunetuzumab efficacy in patients with relapsed or refractory B-cell lymphomas., PMID:39571171
Clinical Pharmacology of Cytokine Release Syndrome with T-Cell-Engaging Bispecific Antibodies: Current Insights and Drug Development Strategies., PMID:39556515
Bispecific antibodies in follicular lymphoma., PMID:39479864
Novel Bispecific T-Cell Engagers for the Treatment of Relapsed B Cell Non-Hodgkin Lymphomas: Current Knowledge and Treatment Considerations., PMID:39479221
Comparative Analysis of Bispecific Antibodies and CAR T-Cell Therapy in Follicular Lymphoma., PMID:39462177
Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies., PMID:39447094
The landscape of T-cell engagers for the treatment of follicular lymphoma., PMID:39398477
[Recent advances in the treatment of DLBCL]., PMID:39358300
A Novel Step-Up Dosage Regimen for Enhancing the Benefit-to-Risk Ratio of Mosunetuzumab in Relapsed or Refractory Follicular Lymphoma., PMID:39328022
Persistent parvovirus B19 infection in a heavily pretreated lymphoma patient receiving mosunetuzumab., PMID:39314008
Safety and Efficacy of Bispecific Antibodies in Adults with Large B-Cell Lymphomas: A Systematic Review of Clinical Trial Data., PMID:39273684
Mosunetuzumab for the treatment of follicular lymphoma., PMID:39259182
The treatment of follicular lymphoma with CD19-directed chimeric antigen receptor T-cell therapy., PMID:38903716
Matching-Adjusted Indirect Comparisons of Axicabtagene Ciloleucel to Mosunetuzumab for the Treatment of Relapsed/Refractory Follicular Lymphoma., PMID:38901633
Cost-effectiveness of treating relapsed or refractory 3L+ follicular lymphoma with axicabtagene ciloleucel vs mosunetuzumab in the United States., PMID:38855109
Bispecific antibodies in the treatment of relapsed/refractory large B-cell lymphoma., PMID:38809821
Population pharmacokinetics and CD20 binding dynamics for mosunetuzumab in relapsed/refractory B-cell non-Hodgkin lymphoma., PMID:38808543
[T-cell recruiting immunotherapies in B-cell lymphoma - the future backbone for all therapy lines?]., PMID:38749439
Cost-effectiveness analysis of mosunetuzumab for treatment of relapsed or refractory follicular lymphoma after two or more lines of systemic therapy in the United States., PMID:38712895
Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?, PMID:38642912