Catalog No.
DHC90715
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
B-lymphocyte surface antigen B1, Membrane-spanning 4-domains subfamily A member 1, MS4A1, Leukocyte surface antigen Leu-16, B-lymphocyte antigen CD20, Bp35, CD20, T3E, T-cell surface antigen T3/Leu-4 epsilon chain, CD3e, CD3E, T-cell surface glycoprotein CD3 epsilon chain
Concentration
1.06 mg/ml
Endotoxin level
Please contact with the lab for this information.
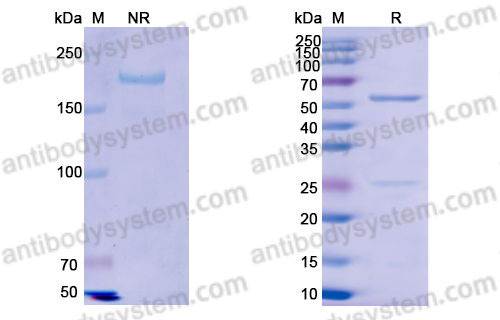
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P11836 & P07766
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Bispecific, REGN-1979, CAS: 1801338-64-6
Clone ID
Odronextamab
Indirect comparison of epcoritamab vs chemoimmunotherapy, mosunetuzumab, or odronextamab in follicular lymphoma., PMID:40472301
CD20×CD3 Bispecific Antibodies in B-NHL: A Review of Translational Science, Pharmacokinetics, Pharmacodynamics, and Dose Strategy in Clinical Research., PMID:40471801
Marginal zone lymphoma with anti-factor H IgM and atypical hemolytic uremic syndrome successfully treated with odronextamab., PMID:40371898
Author Correction: Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial., PMID:40240622
Two strikes, base hit: odronextamab after CAR T cells in LBCL., PMID:40178849
An evaluation of odronextamab for the treatment of multiple subtypes of relapsed/refractory B-cell non-Hodgkin lymphoma., PMID:40106587
Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial., PMID:40097657
[Odronextamab as third line treatment of diffuse large B cells and follicular lymphoma]., PMID:40050189
In vitro comparison of CD20xCD3 bispecific antibodies against diffuse large B-cell lymphoma (DLBCL) cell lines with different levels of expression of CD20., PMID:40032583
New bispecific antibodies in diffuse large B-cell lymphoma., PMID:39911111
Odronextamab monotherapy in R/R DLBCL after progression with CAR T-cell therapy: primary analysis of the ELM-1 study., PMID:39786390
Antibodies to watch in 2025., PMID:39711140
Corrigendum to "Safety and efficacy of odronextamab in patients with relapsed or refractory follicular lymphoma": [Ann Oncol 35 (2024) 1039-1047]., PMID:39627085
Odronextamab: First Approval., PMID:39557796
Bispecific antibodies in follicular lymphoma., PMID:39479864
Novel Bispecific T-Cell Engagers for the Treatment of Relapsed B Cell Non-Hodgkin Lymphomas: Current Knowledge and Treatment Considerations., PMID:39479221
Reply to the Letter to the Editor "Odronextamab against relapsed or refractory follicular lymphoma" by Y. Shimazu., PMID:39442616
The landscape of T-cell engagers for the treatment of follicular lymphoma., PMID:39398477
Safety and Efficacy of Bispecific Antibodies in Adults with Large B-Cell Lymphomas: A Systematic Review of Clinical Trial Data., PMID:39273684
Odronextamab against relapsed or refractory follicular lymphoma., PMID:39236983
Safety and efficacy of odronextamab in patients with relapsed or refractory follicular lymphoma., PMID:39147364
The Evolving Role of Bispecific Antibodies in Diffuse Large B-Cell Lymphoma., PMID:39063920
Bispecific antibodies in the treatment of relapsed/refractory large B-cell lymphoma., PMID:38809821
Molecular assessment of intratumoral immune cell subsets and potential mechanisms of resistance to odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory B-cell non-Hodgkin lymphoma., PMID:38519055
The value of bispecific antibodies in relapsed and refractory DLBCL., PMID:38454535
Antibodies to watch in 2024., PMID:38178784
Bispecific antibodies as monotherapy or in combinations for hematological malignancies: latest updates from the EHA 2023 annual meeting., PMID:37852928
Antibodies to watch in 2023., PMID:36472472
CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models., PMID:36350988
Immuno-PET Monitoring of Lymphocytes Using the CD8-Specific Antibody REGN5054., PMID:35895745
Complete responses to odronextamab in two patients with diffuse large B-cell lymphoma refractory to chimeric antigen receptor T-cell therapy., PMID:35892294
Interference in a Neutralizing Antibody Assay for Odronextamab, a CD20xCD3 Bispecific mAb, from Prior Rituximab Therapy and Possible Mitigation Strategy., PMID:35725847
Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial., PMID:35366963
Management of relapsed or refractory large B-cell lymphoma in patients ineligible for CAR-T cell therapy., PMID:35184664
Bispecific Antibodies for Non-Hodgkin Lymphoma Treatment., PMID:35182296
Translational findings for odronextamab: From preclinical research to a first-in-human study in patients with CD20+ B-cell malignancies., PMID:34997701
The Novel Therapeutic Landscape for Relapsed/Refractory Diffuse Large B Cell Lymphoma., PMID:34922844