Catalog No.
PVV03302
Species reactivity
Influenza A virus (strain A/Hong Kong/5/1983 H3N2) (whales)
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Influenza A virus NP/Nucleo(Met1-Asn498 ).
Tested applications
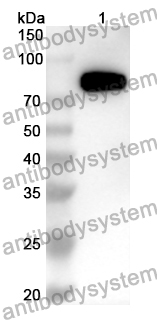
ELISA: 1:4000-1:8000, IHC: 1:50-1:200, WB: 1:1000-1:4000
Target
Nucleoprotein, Nucleocapsid protein, Protein N, NP
Concentration
1.12 mg/ml
Purification
Purified by antigen affinity column.
Accession
P16982
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Comparison of whole blood on filter strips with serum for avian influenza virus antibody detection in wild birds., PMID:40491934
No Evidence of Anti-Influenza Nucleoprotein Antibodies in Retail Milk from Across Canada (April-July 2024)., PMID:40474813
Antigenic Characterization of H1N1 Influenza Viruses That Circulated During the 2019-2020 Season in Philadelphia, Pennsylvania., PMID:40452165
M2e/NP Dual Epitope-Displaying Nanoparticles Enhance Cross-Protection of Recombinant HA Influenza Vaccine: A Universal Boosting Strategy., PMID:40333315
Design, Synthesis and In Vivo Evaluation of a Candidate Fusion Epitopic Construct Vaccine Based on M2e, HA1, HA2, NA and NP Fragments of the Highly Pathogenic Avian H5N1 Influenza Virus., PMID:40256578
Evaluation of safety, immunogenicity, and efficacy of inactivated reverse-genetics-based H5N8 highly pathogenic avian influenza virus vaccine with various adjuvants via parenteral and mucosal routes in chickens., PMID:40181968
Fluorescent Microsphere Immunoassay for Isotype-Specific H5N1 Antibody Detection in Serum and Milk Samples From Dairy Cattle: A Tool for Epidemiological Surveillance., PMID:40145266
Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China., PMID:40141383
Analysis of polyclonal and monoclonal antibody to the influenza virus nucleoprotein in different oligomeric states., PMID:40139568
Detection of antibodies against influenza A viruses in cattle., PMID:40130892
A novel tetravalent influenza vaccine based on one chimpanzee adenoviral vector., PMID:40023902
Adjuvant-free, self-assembling ferritin nanoparticle vaccine coupled with influenza virus hemagglutinin protein carrying M1 and PADRE epitopes elicits cross-protective immune responses., PMID:39958330
Evaluation of an N1 NA antibody-specific enzyme-linked lectin assay for detection of H5N1 highly pathogenic avian influenza virus infection in vaccinated birds., PMID:39956396
Co-Delivery of Multiple Toll-Like Receptor Agonists and Avian Influenza Hemagglutinin on Protein Nanoparticles Enhances Vaccine Immunogenicity and Efficacy., PMID:39924738
Rapid and specific on-site H5Nx avian influenza diagnosis via RPA and PAM-independent CRISPR-Cas12a assay combined with anti-NP antibody-based viral RNA purification., PMID:39896844
Immunogenicity in mice and non-human primates of an Advax-CpG55.2-adjuvanted recombinant hemagglutinin seasonal quadrivalent influenza vaccine., PMID:39798433
Impact of influenza immune imprinting on immune responses to subsequent vaccinations in mice., PMID:39731808
DDO-adjuvanted influenza A virus nucleoprotein mRNA vaccine induces robust humoral and cellular type 1 immune responses and protects mice from challenge., PMID:39692514
Construction with recombinant epitope-expressing baculovirus enhances protective effects of inactivated H9N2 vaccine against heterologous virus., PMID:39671758
Immunoprotective effect of chitosan nanoparticles with different particle sizes against H9N2 avian influenza infection., PMID:39603189
Highly Pathogenic H5N1 Influenza A Virus (IAV) in Blue-Winged Teal in the Mississippi Flyway Is Following the Historic Seasonal Pattern of Low-Pathogenicity IAV in Ducks., PMID:39599570
Avian influenza virus circulation and immunity in a wild urban duck population prior to and during a highly pathogenic H5N1 outbreak., PMID:39578905
DDO-adjuvanted influenza A virus nucleoprotein mRNA vaccine induces robust humoral and cellular type 1 immune responses and protects mice from challenge., PMID:39553933
Deep mutational scanning of H5 hemagglutinin to inform influenza virus surveillance., PMID:39531474
Dissecting immunological mechanisms underlying influenza viral nucleoprotein-induced mucosal immunity against diverse viral strains., PMID:39508450
Inserting CTL Epitopes of the Viral Nucleoprotein to Improve Immunogenicity and Protective Efficacy of Recombinant Protein against Influenza A Virus., PMID:39452110
Differential detection of SARS-CoV-2 variants and influenza A viruses utilizing a dual lateral flow strip based on colloidal gold-labeled monoclonal antibodies., PMID:39341304
Detection and Monitoring of Highly Pathogenic Influenza A Virus 2.3.4.4b Outbreak in Dairy Cattle in the United States., PMID:39339851
The frequency and function of nucleoprotein-specific CD8+ T cells are critical for heterosubtypic immunity against influenza virus infection., PMID:39082839
Efficacy of commercial recombinant HVT vaccines against a North American clade 2.3.4.4b H5N1 highly pathogenic avian influenza virus in chickens., PMID:39012858
Filling two needs with one deed: a combinatory mucosal vaccine against influenza A virus and respiratory syncytial virus., PMID:38975350
An avian-origin internal backbone effectively increases the H5 subtype avian influenza vaccine candidate yield in both chicken embryonated eggs and MDCK cells., PMID:38970848
Evaluation of Efficacy of Surface Coated versus Encapsulated Influenza Antigens in Mannose-Chitosan Nanoparticle-Based Intranasal Vaccine in Swine., PMID:38932376
Systemic prime mucosal boost significantly increases protective efficacy of bivalent RSV influenza viral vectored vaccine., PMID:38926455
A chimeric haemagglutinin-based universal influenza virus vaccine boosts human cellular immune responses directed towards the conserved haemagglutinin stalk domain and the viral nucleoprotein., PMID:38805853
A combination influenza mRNA vaccine candidate provided broad protection against diverse influenza virus challenge., PMID:38805804
Assessment of CD8+ T-cell mediated immunity in an influenza A(H3N2) human challenge model in Belgium: a single centre, randomised, double-blind phase 2 study., PMID:38729196
Cross-protective efficacy and safety of an adenovirus-based universal influenza vaccine expressing nucleoprotein, hemagglutinin, and the ectodomain of matrix protein 2., PMID:38714444
Protective human monoclonal antibodies target conserved sites of vulnerability on the underside of influenza virus neuraminidase., PMID:38430907
The protective effect of intranasal immunization with influenza virus recombinant adenovirus vaccine on mucosal and systemic immune response., PMID:38394888
Gold Nanoparticle-Decorated Polymer Particles for High-Optical-Density Immunoassay Probes., PMID:38291580
Thiolated chitosan encapsulation constituted mucoadhesive nanovaccine confers broad protection against divergent influenza A viruses., PMID:38220319
Harnessing preexisting influenza virus-specific immunity increases antibody responses against SARS-CoV-2., PMID:38206036
A Multiseason Randomized Controlled Trial of Advax-Adjuvanted Seasonal Influenza Vaccine in Participants With Chronic Disease or Older Age., PMID:38157402
Equine influenza outbreak in Eastern of Algeria in 2021: The first introduction of Florida Clade 1 to Maghreb area., PMID:38118336
Combinatorial immune refocusing within the influenza hemagglutinin RBD improves cross-neutralizing antibody responses., PMID:38096052
Antibody Responses to Influenza Vaccination are Diminished in Patients With Inflammatory Bowel Disease on Infliximab or Tofacitinib., PMID:37941436
Efficacy of inactivated and RNA particle vaccines against a North American Clade 2.3.4.4b H5 highly pathogenic avian influenza virus in chickens., PMID:37932132
Characterization of non-neutralizing human monoclonal antibodies that target the M1 and NP of influenza A viruses., PMID:37916834
Nanoparticles Carrying Conserved Regions of Influenza A Hemagglutinin, Nucleoprotein, and M2 Protein Elicit a Strong Humoral and T Cell Immune Response and Protect Animals from Infection., PMID:37764217