Catalog No.
DHD23002
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
Fusion - ALB (albumin, human serum albumin, HSA) 25-609 - IFNA2 (interferon alpha 2) *b (R46,H57) 24-188
Target
IFNAR1/IFNAR2
Concentration
0.5 mg/ml
Endotoxin level
Please contact with the lab for this information.
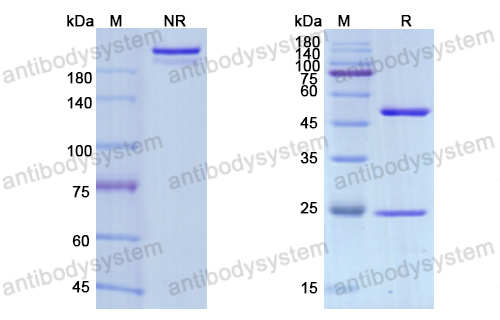
Purity
>95% as determined by SDS-PAGE.
Purification
Purified by Ion Exchange Chromatography.
Accession
P17181, P48551
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Alb-IFNA2 R23 (608), Albumin- interferon alpha-2b, Joulferon ZALBIN™
Clone ID
Albinterferon alfa-2b
Randomized, controlled pharmacokinetic and pharmacodynamic evaluation of albinterferon in patients with chronic hepatitis B infection., PMID:24995515
Albinterferon α2b adsorption to silicone oil-water interfaces: effects on protein conformation, aggregation, and subvisible particle formation., PMID:24382812
Comparative efficacy, pharmacokinetic, pharmacodynamic activity, and interferon stimulated gene expression of different interferon formulations in HIV/HCV genotype-1 infected patients., PMID:24166150
Decline in pulmonary function during chronic hepatitis C virus therapy with modified interferon alfa and ribavirin., PMID:23490379
Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies., PMID:23470616
Protein covalent dimer formation induced by reversed-phase HPLC conditions., PMID:23280771
Effects of solution conditions on methionine oxidation in albinterferon alfa-2b and the role of oxidation in its conformation and aggregation., PMID:23203978
Randomized trial of albinterferon alfa-2b every 4 weeks for chronic hepatitis C virus genotype 2/3., PMID:22863266
Physical stability of albinterferon-α(2b) in aqueous solution: effects of conformational stability and colloidal stability on aggregation., PMID:22674119
Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: impaired viral kinetics and therapeutic response., PMID:22331604
Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner., PMID:22318926
FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus., PMID:22147963
Characterization of the self-association of human interferon-α2b, albinterferon-α2b, and pegasys., PMID:21975852
Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3., PMID:21873466
Demonstrating the stability of albinterferon alfa-2b in the presence of silicone oil., PMID:21780119
Population pharmacokinetics and exposure-response of albinterferon alfa-2b., PMID:21551316
Insulin resistance is independently associated with significant hepatic fibrosis in Asian chronic hepatitis C genotype 2 or 3 patients., PMID:21410752
Single-dose pharmacokinetics, safety, and tolerability of albinterferon alfa-2b in subjects with end-stage renal disease on hemodialysis compared to those in matched healthy volunteers., PMID:21098255
Albinterferon-alpha 2b: a new treatment option for hepatitis C., PMID:20828335
Why do we need another interferon?, PMID:20800652
Albinterferon Alfa-2b was not inferior to pegylated interferon-α in a randomized trial of patients with chronic hepatitis C virus genotype 2 or 3., PMID:20600017
Albinterferon Alfa-2b was not inferior to pegylated interferon-α in a randomized trial of patients with chronic hepatitis C virus genotype 1., PMID:20600013
28th Annual JPMorgan Healthcare Conference--Human Genome Sciences and Celgene., PMID:20191423
Hepatitis C: The role of new interferons in the era of STAT-C., PMID:19713984
Positive and negative prediction of sustained virologic response at weeks 2 and 4 of treatment with albinterferon alfa-2b or peginterferon alfa-2a in treatment-naïve patients with genotype 1, chronic hepatitis C., PMID:19447518
Changes in B-lymphocyte stimulator protein levels during treatment with albinterferon alfa-2b in patients with chronic hepatitis C who have failed previous interferon therapy., PMID:20849568
Early prediction of sustained virological response at day 3 of treatment with albinterferon-alpha-2b in patients with genotype 2/3 chronic hepatitis C., PMID:19291180
Albinterferon alfa-2b, a novel fusion protein of human albumin and human interferon alfa-2b, for chronic hepatitis C., PMID:19275518
Gateways to Clinical Trials., PMID:19229381
An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C., PMID:19175870
Gateways to clinical trials., PMID:19088949
Safety and antiviral activity of albinterferon alfa-2b in prior interferon nonresponders with chronic hepatitis C., PMID:19061971
Albinterferon alfa-2b dosed every two or four weeks in interferon-naïve patients with genotype 1 chronic hepatitis C., PMID:18666223
Safety and antiviral activity of albinterferon alfa-2b dosed every four weeks in genotype 2/3 chronic hepatitis C patients., PMID:18467185
Gateways to clinical trials., PMID:18200333
Gateways to clinical trials., PMID:17982511
Anti-hepatitis C virus activity of albinterferon alfa-2b in cell culture., PMID:17573950