Catalog No.
DHB95904
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
T-cell surface antigen T4/Leu-3, T-cell surface glycoprotein CD4, CD4
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
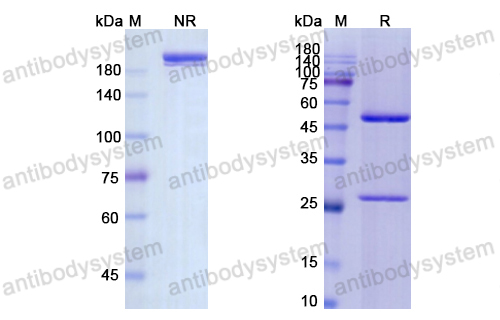
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01730
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ORTHOCLONE OKT4 A, CAS: 156586-90-2
Clone ID
Cedelizumab
Vedolizumab as induction and maintenance therapy for ulcerative colitis, PMID: 23964932
Long-term safety of vedolizumab for inflammatory bowel disease, PMID: 32876349
Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis, PMID: 31470005
Vedolizumab as induction and maintenance therapy for Crohn's disease, PMID: 23964933
The safety of vedolizumab for the treatment of ulcerative colitis, PMID: 28276855
Safety of New Biologics (Vedolizumab and Ustekinumab) and Small Molecules (Tofacitinib) During Pregnancy: A Review, PMID: 32562207
The safety of vedolizumab for ulcerative colitis and Crohn's disease, PMID: 26893500
Long-term Efficacy of Vedolizumab for Crohn's Disease, PMID: 27683798
Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview, PMID: 31064370
Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis, PMID: 28204866
A Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Vedolizumab, PMID: 28523450
Vedolizumab Therapy in Severe Pediatric Inflammatory Bowel Disease, PMID: 27598742
Endoscopic, Radiologic, and Histologic Healing With Vedolizumab in Patients With Active Crohn's Disease, PMID: 31279871
Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn's disease, PMID: 32656800
Long-term Efficacy of Vedolizumab for Ulcerative Colitis, PMID: 27683800
Therapeutic Drug Monitoring With Ustekinumab and Vedolizumab in Inflammatory Bowel Disease, PMID: 29788272
Vedolizumab Induces Endoscopic and Histologic Remission in Patients With Crohn's Disease, PMID: 31175865
Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease, PMID: 29430672
Effects of Vedolizumab Therapy on Extraintestinal Manifestations in Inflammatory Bowel Disease, PMID: 29484571
Vedolizumab in the Perioperative Management of Inflammatory Bowel Disease, PMID: 30914021
Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn's disease patients with prior failure to anti-TNF treatment, PMID: 32441396
Safety and Effectiveness of Vedolizumab in Patients with Steroid-Refractory Gastrointestinal Acute Graft-versus-Host Disease: A Retrospective Record Review, PMID: 30468919
Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study, PMID: 30807613
Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice, PMID: 31777929
Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease, PMID: 29730603
Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review, PMID: 31076751
Vedolizumab and etrolizumab for ulcerative colitis: twins or simple cousins?, PMID: 31951748
An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab, PMID: 27252400
Vedolizumab for perianal Crohn's disease: a multicentre cohort study in 151 patients, PMID: 32080886
Vedolizumab treatment persistence and safety in a 2-year data analysis of an extended access programme, PMID: 33210333
Vedolizumab for the treatment of Crohn's disease, PMID: 29406811
Vedolizumab use after failure of TNF-α antagonists in children and adolescents with inflammatory bowel disease, PMID: 30219028
Rapid Response to Vedolizumab Therapy in Biologic-Naive Patients With Inflammatory Bowel Diseases, PMID: 29857145
Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data, PMID: 31747086
The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn's disease refractory to anti-tumour necrosis factor, PMID: 32249966
A product review of vedolizumab in inflammatory bowel disease, PMID: 30897022
Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study, PMID: 33345331
Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2, PMID: 29471381
Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study, PMID: 30518410
Development and Validation of Clinical Scoring Tool to Predict Outcomes of Treatment With Vedolizumab in Patients With Ulcerative Colitis, PMID: 32062041
Should we use vedolizumab as mono or combo therapy in ulcerative colitis?, PMID: 30060935
Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease, PMID: 25996351
Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure, PMID: 32981127
Drug survival of anti-TNF agents compared with vedolizumab as a second-line biological treatment in inflammatory bowel disease: results from nationwide Swedish registers, PMID: 33340426
Benefit-Risk Assessment of Vedolizumab in the Treatment of Crohn's Disease and Ulcerative Colitis, PMID: 30830573
Therapeutic drug monitoring with vedolizumab in inflammatory bowel disease, PMID: 31646853
Vedolizumab for the treatment of inflammatory bowel diseases: from symptomatic control to mucosal healing, PMID: 30860423
Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients With Crohn's Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis, PMID: 29935327
Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease-a prospective multicentre cohort study, PMID: 32901983
Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn's Disease Patients Stratified by Age, PMID: 28070861