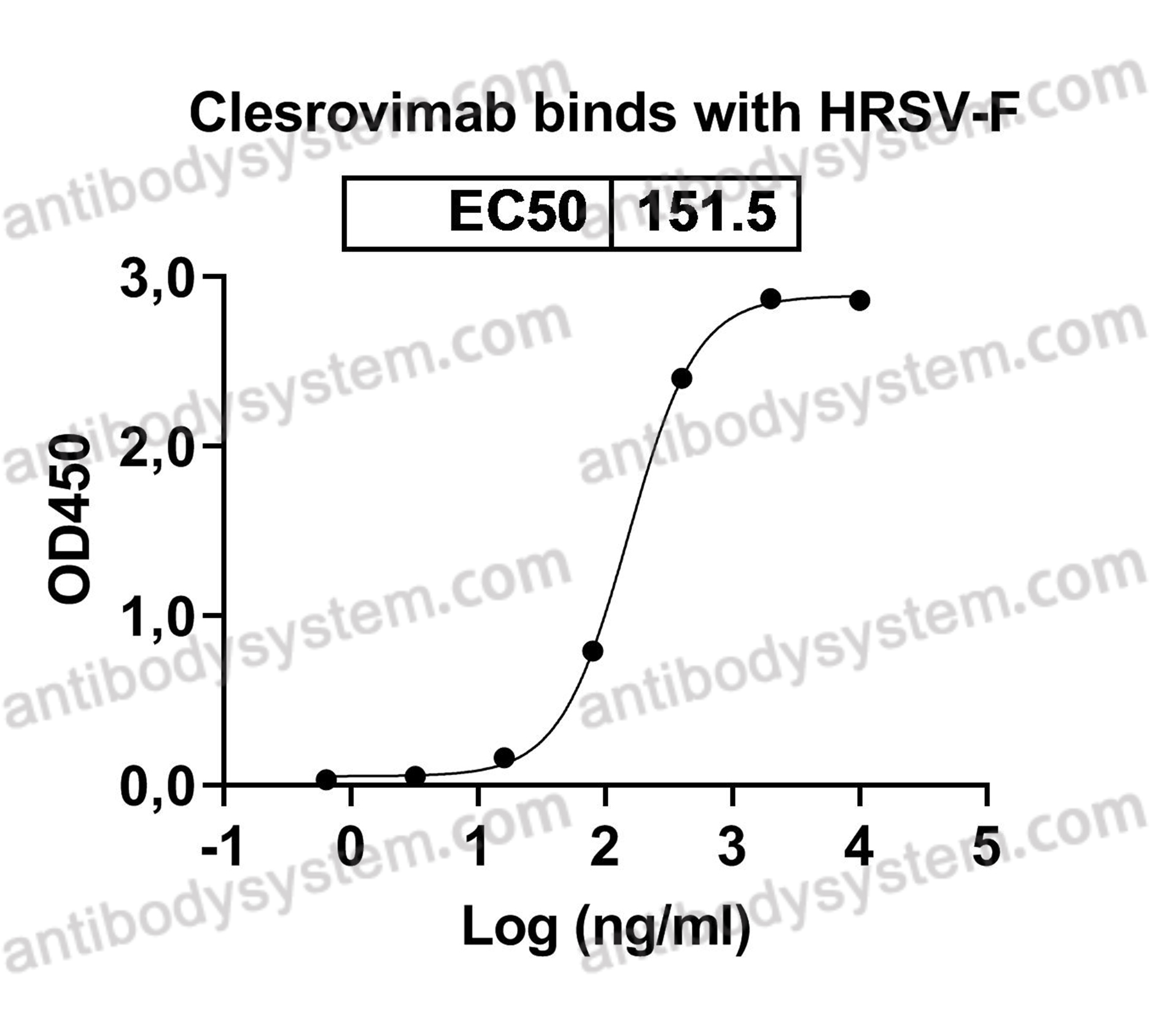
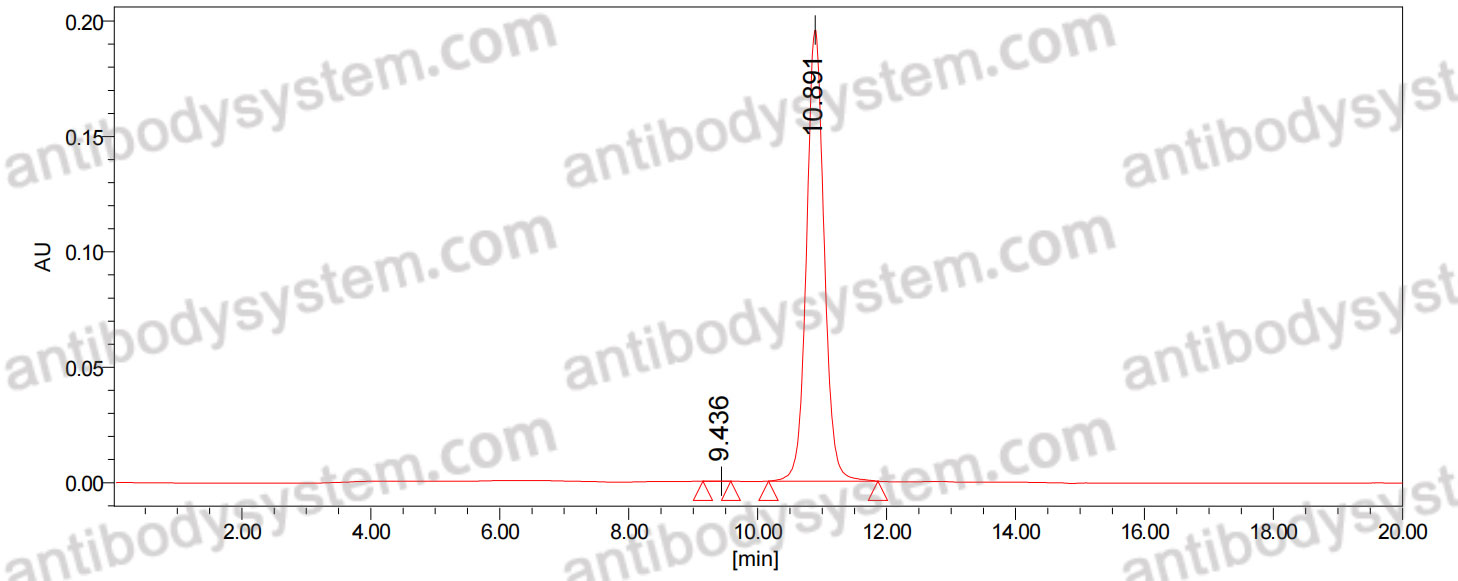
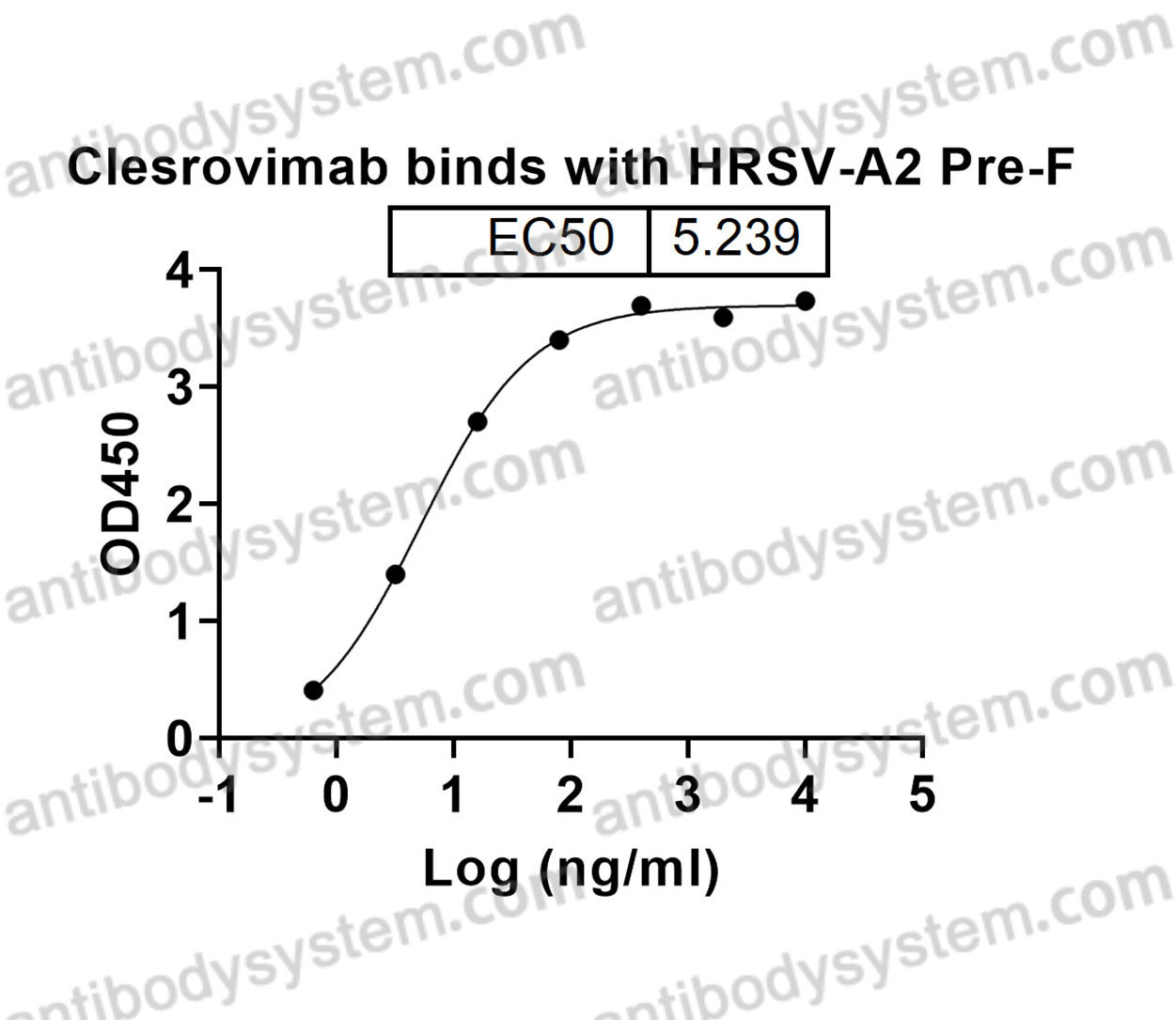
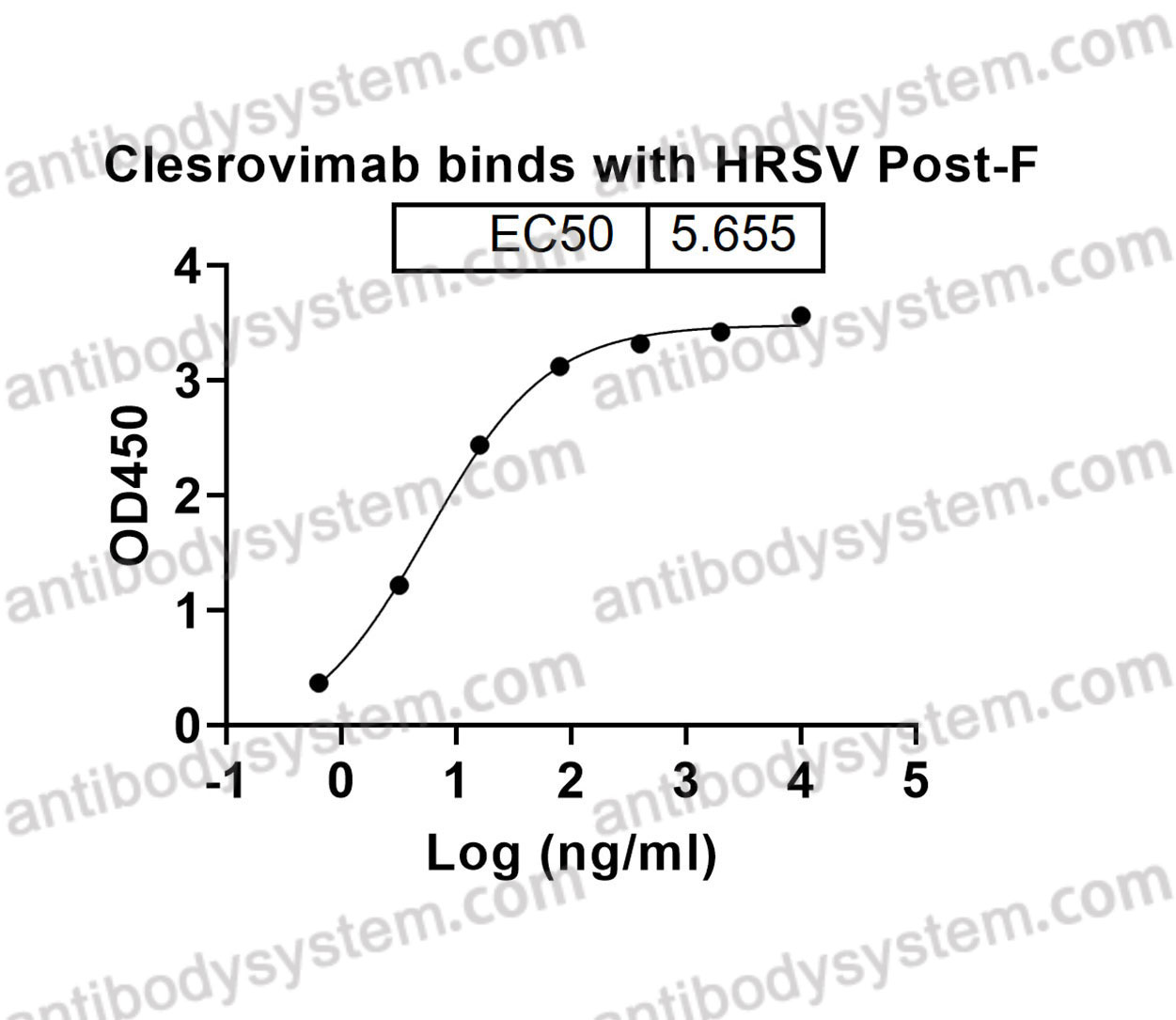
Catalog No.
DVV02808
Expression system
Mammalian Cells
Species reactivity
RSV (respiratory syncytial virus)
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
F, Fusion glycoprotein F0, Fusion glycoprotein F2, p27, Intervening segment, Pep27, Peptide 27, Fusion glycoprotein F1
Concentration
3.74 mg/ml
Endotoxin level
Please contact with the lab for this information.
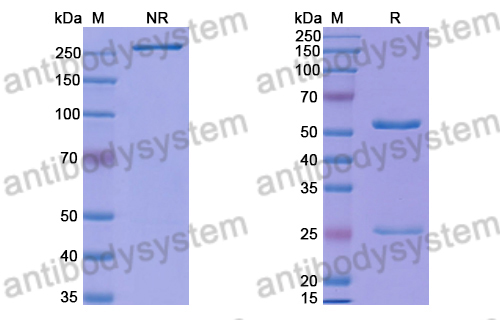
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P03420
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MK 1654, MK-1654, MK1654, CAS: 2429913-18-6
Clone ID
Clesrovimab
Supporting Pediatric RSV Clinical Trials Through Close Epidemiological Surveillance During the SARS-CoV-2 Pandemic., PMID:40312764
Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection., PMID:40262853
Efficacy of monoclonal antibodies and maternal vaccination for prophylaxis of respiratory syncytial virus disease., PMID:40240559
Anti-Idiotypic Antibody as a Booster Vaccine Against Respiratory Syncytial Virus., PMID:39852814
The recent landscape of RSV vaccine research., PMID:39802673
A Phase 1b/2a Trial of a Half-life Extended Respiratory Syncytial Virus Neutralizing Antibody, Clesrovimab, in Healthy Preterm and Full-term Infants., PMID:39601265
Development of High-Titer Antidrug Antibodies in a Phase 1b/2a Infant Clesrovimab Trial Are Associated With RSV Exposure Beyond Day 150., PMID:39590882
Mind the (preterm) gap: inequality in the UK's current RSV immunisation approach will leave many preterm babies unprotected against RSV this winter., PMID:39379140
Systematic Review of the Efficacy and Safety of RSV-Specific Monoclonal Antibodies and Antivirals in Development., PMID:39209729
Genomic evolution of human respiratory syncytial virus during a decade (2013-2023): bridging the path to monoclonal antibody surveillance., PMID:38588960
[Prevention of respiratory syncytial virus infection in infants. What has been done and where are we today?]., PMID:38329302
Quantification of clesrovimab, an investigational, half-life extended, anti-respiratory syncytial virus protein F human monoclonal antibody in the nasal epithelial lining fluid of healthy adults., PMID:37976891
Development of mRNA vaccines against respiratory syncytial virus (RSV)., PMID:36280532