Catalog No.
DHJ63102
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
SGRF, IL-23-A, IL-23 subunit alpha, Interleukin-23 subunit p19, IL23A, Interleukin-23 subunit alpha, IL-23p19
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
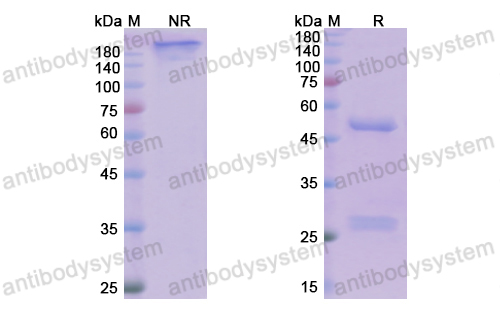
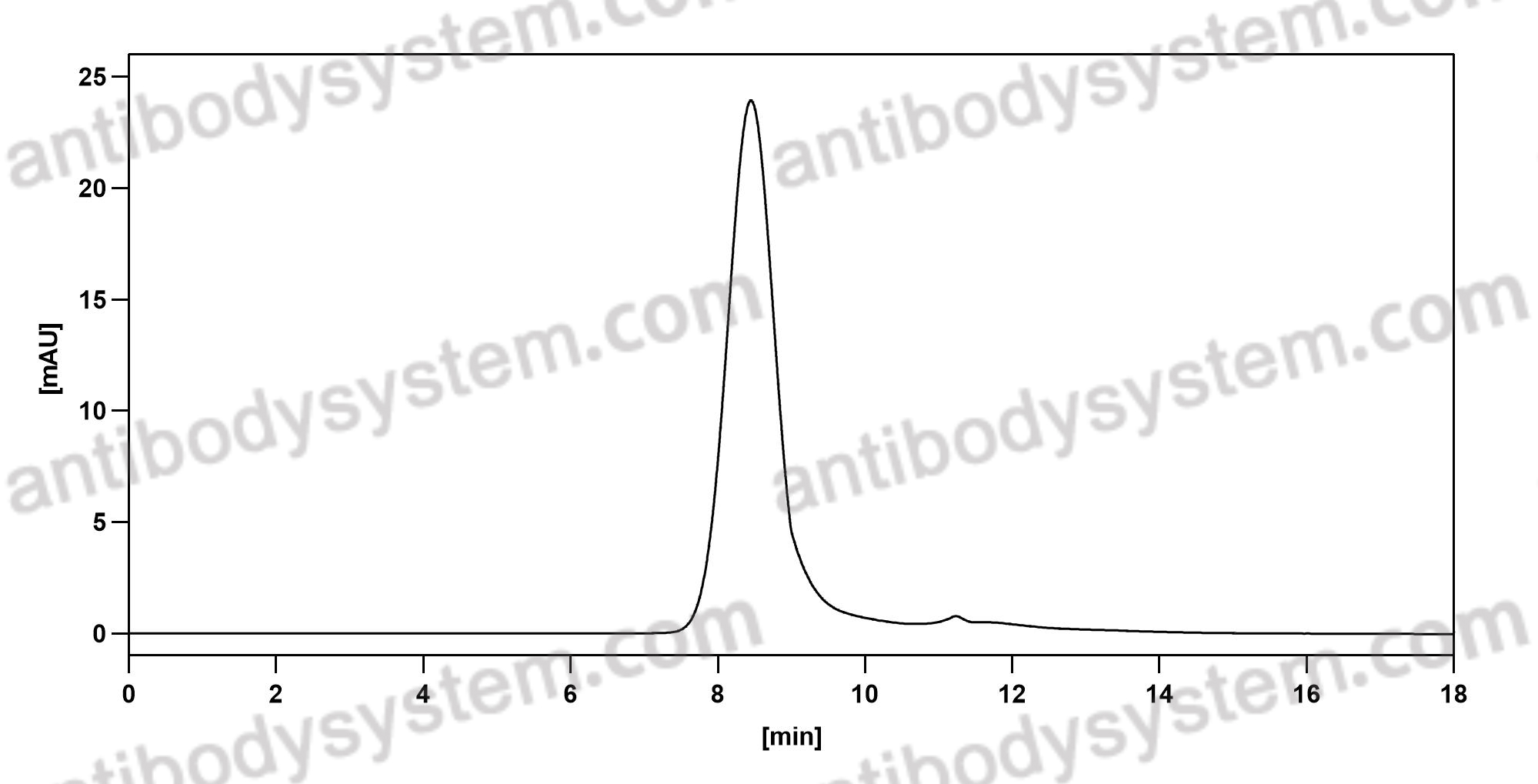
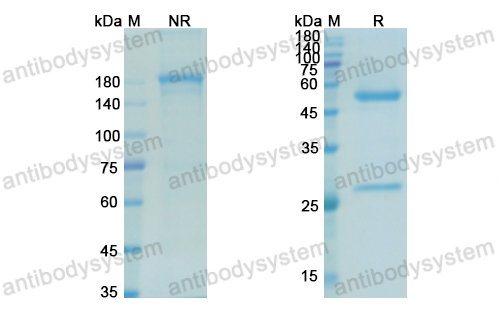
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NPF7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MK-3222, SCH900222, CAS: 1326244-10-3
Clone ID
Tildrakizumab
Comprehensive Post-marketing safety analysis of Tildrakizumab: insights from the FDA adverse event Reporting system., PMID:40512376
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Tildrakizumab Treatment Patterns in Adults With Moderate-to-Severe Plaque Psoriasis: A Retrospective Analysis From the Canadian Patient Support Program., PMID:40442565
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use., PMID:40430215
Effectiveness of Tildrakizumab 200 mg in Moderate-to-Severe Plaque Psoriasis: A Multicenter Real-World Study Analyzing Patient Outcomes by Weight, PASI, BMI, and Previous Therapies., PMID:40413278
Age of Onset Matters: Tildrakizumab Response in Early vs Late-Onset Psoriasis., PMID:40406970
Interleukin-23p19 inhibitors for the treatment of moderate-to-severe psoriasis: an expert opinion of real-world evidence studies in Europe., PMID:40396610
Complete Resolution of Residual Psoriasis by Adding Tapinarof Cream to Tildrakizumab: A Case Report., PMID:40391769
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Efficacy and safety of tildrakizumab in patients with early- vs late-onset psoriasis., PMID:40365708
Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis., PMID:40329054
Optimizing Tildrakizumab Dosing in Psoriasis: A 52-Week Multicenter Retrospective Study Comparing 100 mg and 200 mg-IL PSO (Italian Landscape Psoriasis)., PMID:40266488
Frontal Fibrosing Alopecia: A Comprehensive Review with Recent Updates., PMID:40162350
Efficacy of tildrakizumab 200 mg for treating difficult-to-treat patient populations with moderate-to-severe plaque psoriasis., PMID:40047295
Safety of Interleukin Inhibitors in Psoriatic Patients with Latent Tuberculosis Infection Without Chemoprophylaxis: A Systematic Review., PMID:40026108
Tildrakizumab in the treatment of plaque psoriasis in an HIV+ patient: a case report and literature review of anti-interleukin drugs., PMID:39969048
Real-Life Experience with Tildrakizumab in Plaque Psoriasis with Palmoplantar Involvement: A Multi-Center Retrospective Italian Study., PMID:39847259
Adverse events associated with IL-23 and IL-12/23 inhibitors in the clinical management of psoriasis: a comprehensive pharmacovigilance analysis., PMID:39833957
Tildrakizumab and Quality of Life: Deep Dive into the Impact of Psoriasis and Treatment on Different Domains-Should Psychosocial Life Impairment Be Considered a Comorbidity?, PMID:39797306
Editor's Highlights-February 2025., PMID:39791934
Demographics, Disease Characteristics, and Treatment Patterns of Patients with Plaque Psoriasis Treated with Biological Drugs: The Experience of a Single-Centre Study in Poland., PMID:39768570
INDIVIDUAL ARTICLE: Psoriasis and Obesity: Optimizing Pharmacologic Treatment and Lifestyle Interventions., PMID:39761151
Efficacy and safety of tildrakizumab for the treatment of moderate-to-severe plaque psoriasis of the scalp: Week 52 results from a phase 3b, randomized, double-blind, placebo-controlled trial., PMID:39722400
Real-World Effectiveness of Tildrakizumab for Moderate-to-Severe Plaque Psoriasis in Canada., PMID:39673433
Off-label dermatologic uses of IL-23 inhibitors., PMID:39647840
Successful treatment of psoriasis and lichen planus with tildrakizumab., PMID:39644480
Biologics Use for Psoriasis during Pregnancy and Its Related Adverse Outcomes in Pregnant Women and Newborns: Findings from WHO Pharmacovigilance Study., PMID:39626647
Facial psoriasis treated with tildrakizumab., PMID:39611417
Tildrakizumab Treatment for Psoriasis in Real-world Practice: An Analysis from the Swiss Registry (SDNTT)., PMID:39601368
The Clinical and Molecular Response of Pyoderma Gangrenosum to IL-23 Blockade: Result from a Proof-of-Concept Open-Label Clinical Trial., PMID:39547392
Indirect comparison of deucravacitinib and other systemic treatments for moderate to severe plaque psoriasis in Asian populations: A systematic literature review and network meta-analysis., PMID:39526612
Effectiveness of tildrakizumab 200 mg: an Italian multicenter study., PMID:39462515
Prescribing Pattern and Safety Profile of Biological Agents for Psoriasis in Real-World Practice: A Four-Year Calabrian Pharmacovigilance Analysis., PMID:39458658
A prospective Real-Life Multicenter Study of Tildrakizumab 200 mg in Patients with Moderate-Severe Psoriasis: Who is the Ideal Patient?, PMID:39453881
Off-Label Use of Tildrakizumab in Patients with Hidradenitis Suppurativa., PMID:39450621
Real-World Effectiveness and Safety of Tildrakizumab for Moderate-to-Severe Plaque Psoriasis in Adult Patients: A 52-Week Multicenter Retrospective Study., PMID:39450612
Improvement in Patient-reported Symptoms and Satisfaction with Tildrakizumab in a Real-world Study in Patients with Moderate-to-severe Plaque Psoriasis., PMID:39445319
Long-Standing Remission After Tildrakizumab Treatment in a Case of Refractory Type I Pityriasis Rubra Pilaris in a Breast Cancer Patient., PMID:39430645
Impact of tildrakizumab on the quality of life of patients with moderate-to-severe psoriasis: a 36-week prospective monocentric real-life observational study., PMID:39400048
Patients' Preferences Regarding Modes of Systemic Psoriasis Treatment - A Qualitative Study., PMID:39381162
Comparison table: Some drugs for plaque psoriasis., PMID:39302349
Drugs for plaque psoriasis., PMID:39302348
Corrigendum to Tildrakizumab Inadequate Responders Switching to an Alternative IL-23 Inhibitor: A Case Series., PMID:39295833
Corrigendum to Retrospective Analysis in Patients With Moderate to Severe Plaque Psoriasis Treated With Tildrakizumab: Real-Life Clinical Data., PMID:39295829
Uncovering the Differences: How DLQI and WHO-5 Scores Vary in Moderate-to-Severe Psoriasis Patients Treated with Tildrakizumab 100 mg vs. 200 mg?, PMID:39274452
The immunologic role of IL-23 in psoriatic arthritis: a potential therapeutic target., PMID:39230202
A case of eczematous paradoxical reactions on the extremities during tildrakizumab treatment for plaque psoriasis., PMID:39229699
Effectiveness and safety of tildrakizumab in individuals with HIV and psoriasis: A case series., PMID:39226448