Catalog No.
DXX00301
Expression system
Mammalian Cells
Species reactivity
Clostridioides difficile
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Toxin B, 3.4.22.-, Glucosyltransferase TcdB, 2.4.1.-, tcdB, toxB
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
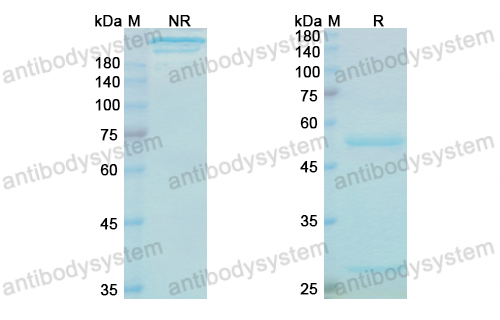
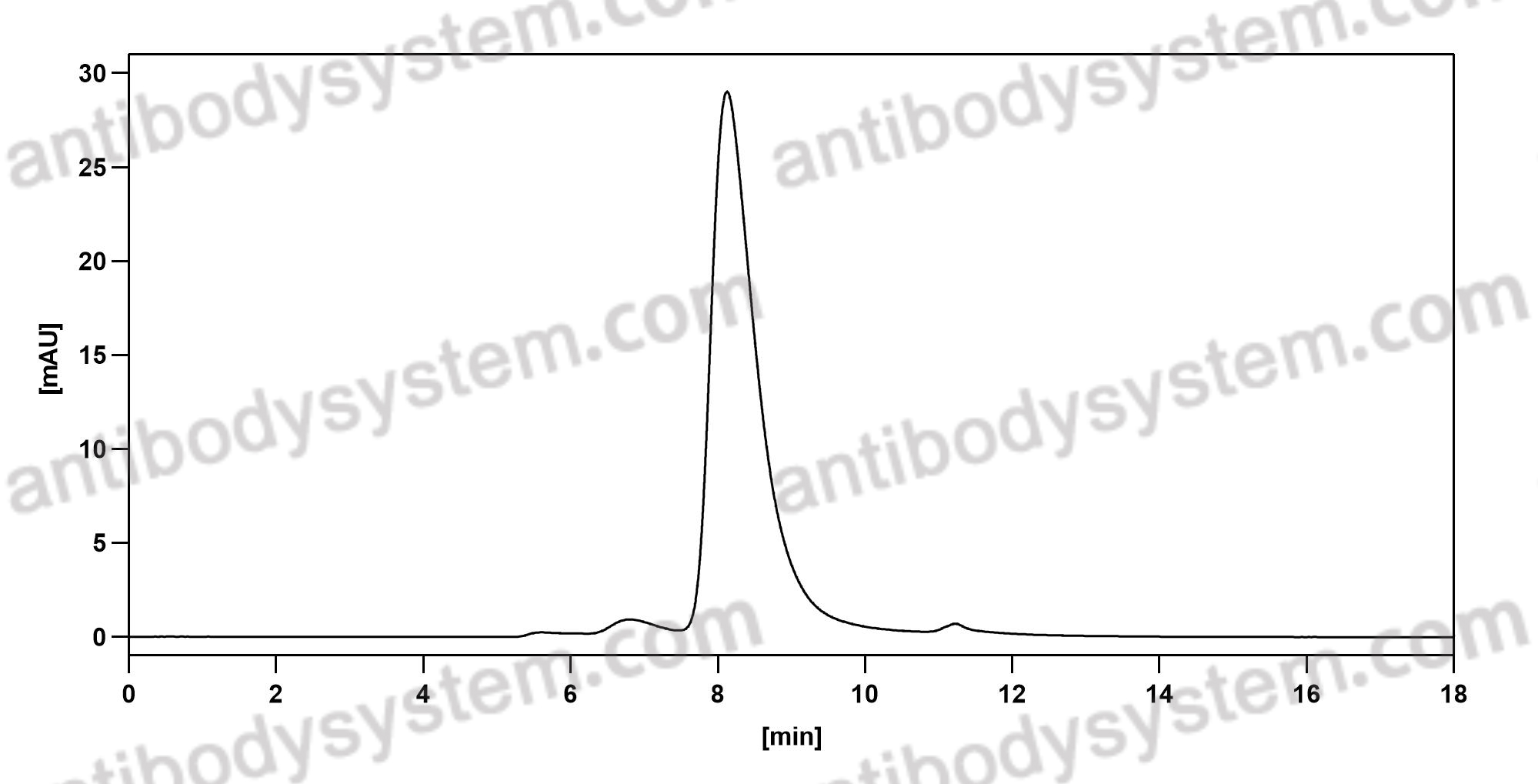
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P18177
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MK-6072, CDB-1, MDX-1388, MK-3415A (combination of actoxumab and bezlotoxumab), formerly CDB 1, CAS: 1246264-45-8
Clone ID
Bezlotoxumab
Development of a toxin-selective immunotracer for in vivo detection of Clostridioides difficile infection by immunoPET., PMID:40512279
Extended-pulsed fidaxomicin therapy for recurrent Clostridioides difficile infection after standard vancomycin and fidaxomicin failure: A case report., PMID:40250802
Evaluating Bezlotoxumab-Fidaxomicin Combination Therapy in Clostridioides Infection: A Single-Center Retrospective Study from Aichi Prefecture, Japan., PMID:40149040
Characteristics and Real-World Outcomes of Patients Treated with Fecal Microbiota, Live-jslm (RBL) for the Prevention of Recurrent Clostridioides difficile Infection., PMID:40119029
Impact of adjunct bezlotoxumab for preventing Clostridioides difficile infection recurrence in patients post - hematopoietic stem cell transplantation., PMID:39865797
Management of Recurrent Clostridioides difficile Infection (rCDI): A Systematic Literature Review to Assess the Feasibility of Indirect Treatment Comparison (ITC)., PMID:39821840
Arrhythmic storm in a liver transplant recipient: Could bezlotoxumab be the trigger?, PMID:39701915
Oral Capsule FMT Combined With Bezlotoxumab Is a Successful Rescue Protocol Following Failure of FMT Alone in the Treatment of Recurrent C. difficile Infection., PMID:39621384
[Evaluation of the management of Clostridioides difficile infection as a risk factor for recurrence. A retrospective observational study]., PMID:39588562
The always evolving diagnosis and management of Clostridioides difficile colitis: What you need to know., PMID:39509684
Microbiota restoration for recurrent Clostridioides difficile infection., PMID:39382853
[Clostridioides difficile infections: Update and therapeutic guidelines]., PMID:39377303
Recurrent Clostridioides difficile infections in solid organ transplant recipients: The international CALIPSO study., PMID:39374859
Fidaxomicin versus oral vancomycin for Clostridioides difficile infection among patients at high risk for recurrence based on real-world experience., PMID:39363592
Clostridioides difficile infection: an update., PMID:39282548
Therapeutics involved in managing initial and recurrent Clostridium difficile infection: An updated literature review., PMID:39281262
Diagnostic and therapeutic management of Clostridioides difficile infection., PMID:39271443
Diagnosis and Management of Clostridioides difficile in Inflammatory Bowel Disease., PMID:39230037
Is advanced age still a risk factor for recurrence of C. difficile infection in the era of new treatments?, PMID:39141079
Treating Helicobacter pylori and Recurrent Clostridioides difficile Coinfection: A Delicate Balance in Management and a Need for Guidelines., PMID:38835648
Exploring the Toxin-Mediated Mechanisms in Clostridioides difficile Infection., PMID:38792835
Breaking the Cycle of Recurrent Clostridioides difficile Infections: A Narrative Review Exploring Current and Novel Therapeutic Strategies., PMID:38739837
Fighting against Clostridioides difficile infection: Current medications., PMID:38734214
Clostridioides Difficile: A Concise Review of Best Practices and Updates., PMID:38726585
Monoclonal antibody-mediated neutralization of Clostridioides difficile toxin does not diminish induction of the protective innate immune response to infection., PMID:38701911
Clostridioides difficile Colitis., PMID:38677819
Management and Outcomes of Patients at a Specialty Clinic for Clostridioides difficile Infection., PMID:38665169
New treatment approaches for Clostridioides difficile infections: alternatives to antibiotics and fecal microbiota transplantation., PMID:38591915
Clostridioides difficile infection: a changing treatment paradigm., PMID:38571533
Live Biotherapeutic Products for the Prevention of Recurrent Clostridioides difficile Infection., PMID:38546138
Enhancing bezlotoxumab binding to C. difficile toxin B2: insights from computational simulations and mutational analyses for antibody design., PMID:38511411
Management of Clostridioides difficile Infection: Diagnosis, Treatment, and Future Perspectives., PMID:38508330
Outcomes After Fecal Microbiota Transplantation in Combination With Bezlotoxumab for Inflammatory Bowel Disease and Recurrent Clostridioides difficile Infection., PMID:38501667
The management of Clostridioides difficile infection: from empirism to evidence., PMID:38344334
Structural and functional insight into the interaction of Clostridioides difficile toxin B and FZD7., PMID:38308843
Bezlotoxumab during the first episode of Clostridioides difficile infection in patients at high risk of recurrence., PMID:38236366
European Practice for CDI Treatment., PMID:38175471
Efficacy of bezlotoxumab to prevent recurrent Clostridioides difficile infection (CDI) in patients with multiple prior recurrent CDI., PMID:37931679
Clostridioides difficile infection in the allogeneic hematopoietic cell transplant recipient., PMID:37787395
Biofilm Formation of Clostridioides difficile, Toxin Production and Alternatives to Conventional Antibiotics in the Treatment of CDI., PMID:37764005
Efficacy of Bezlotoxumab Against Clostridioides difficile Infection: A Case-Series Study at a University Hospital in Japan and Literature Review., PMID:37664309
Therapeutics for Clostridioides difficile infection: molecules and microbes., PMID:37606962
How Would You Manage This Patient With Clostridioides difficile Infection? : Grand Rounds Discussion From Beth Israel Deaconess Medical Center., PMID:37549387
Prevention and treatment of C. difficile in cancer patients., PMID:37527003
Efficacy, Safety, and Cost-effectiveness of Bezlotoxumab in Preventing Recurrent Clostridioides difficile Infection : Systematic Review and Meta-analysis., PMID:37395627
Double-Blind, Placebo-Controlled Study of Bezlotoxumab in Children Receiving Antibacterial Treatment for Clostridioides difficile Infection (MODIFY III)., PMID:37389891
Clostridium difficile in inflammatory bowel disease., PMID:37265220
[Clostridioides difficile - New Insights and Therapy Recommendations]., PMID:37257477
Clostridioides difficile Infection in an Italian Tertiary Care University Hospital: A Retrospective Analysis., PMID:37237740