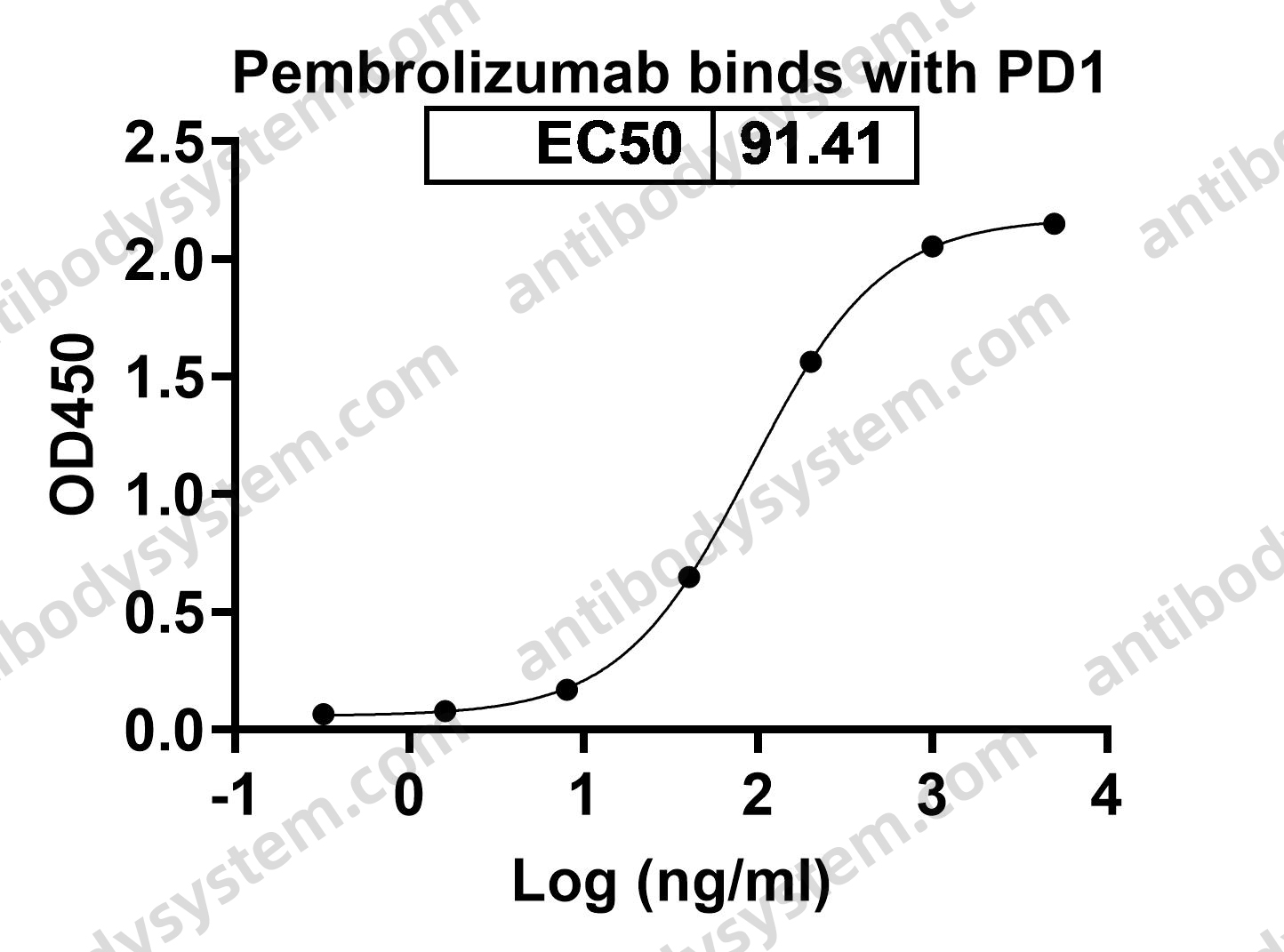
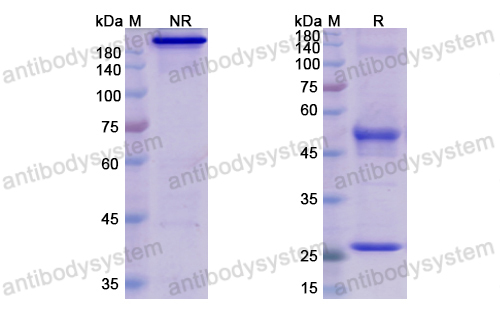
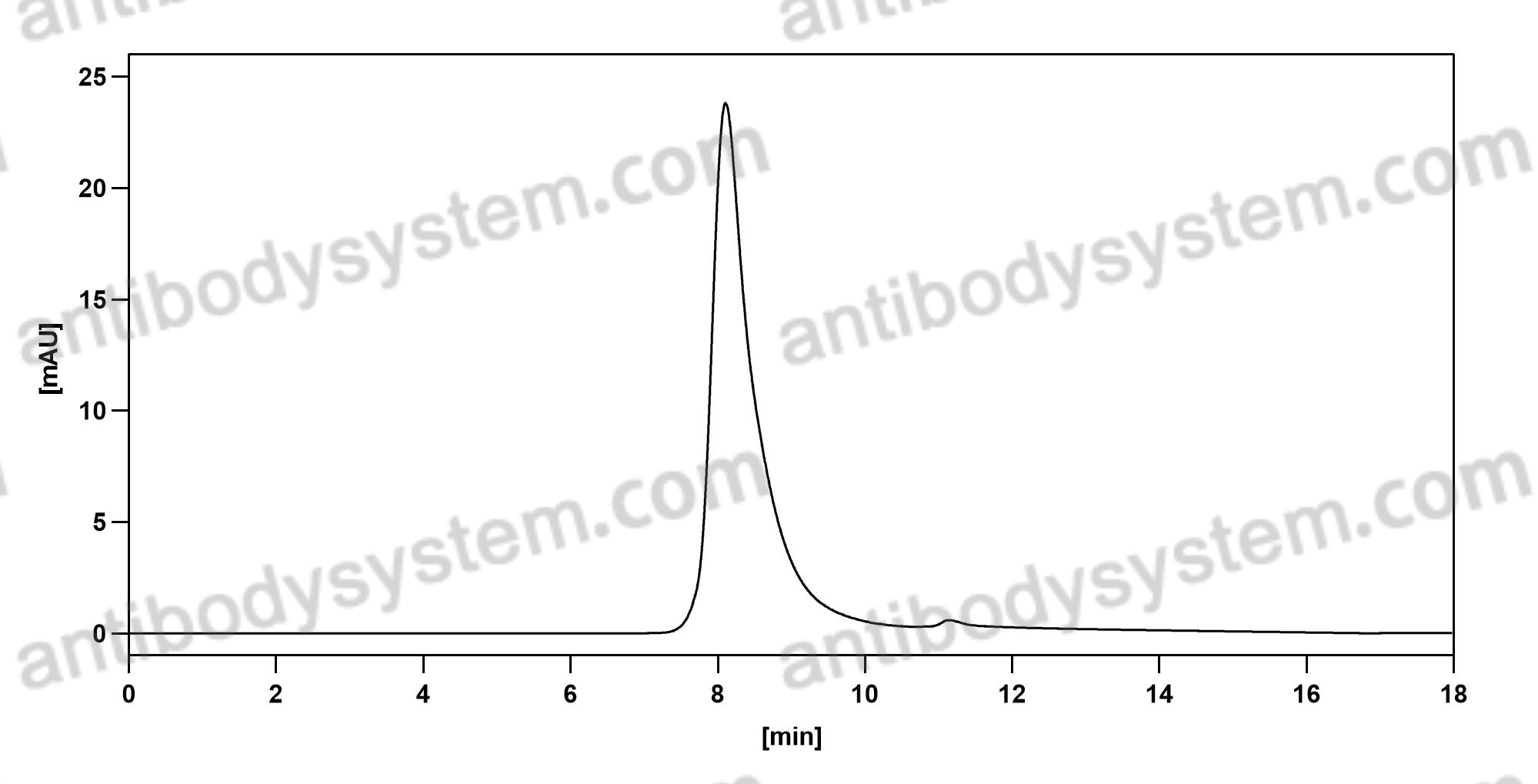
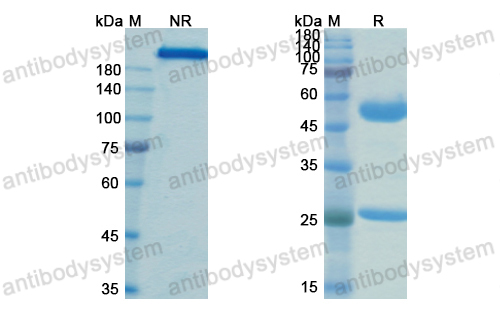
Neoadjuvant dose-dense anthracycline and cyclophosphamide in combination with carboplatin, paclitaxel, and pembrolizumab for triple-negative breast cancer: a systematic review and meta-analysis., PMID:40512324
LiGeR-HN phase III trials of petosemtamab + pembrolizumab and petosemtamab monotherapy in recurrent or metastatic HNSCC., PMID:40511820
Pembrolizumab-induced myocarditis, myositis, and myasthenia gravis overlap syndrome (IM3OS) treated with Efgartigimod: case report., PMID:40510022
Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update., PMID:40507360
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy., PMID:40507249
Pembrolizumab plus chemotherapy in advanced endometrial cancer: a cost-effectiveness analysis., PMID:40506696
[Lichen sclerosus induced by pembrolizumab]., PMID:40506272
Management of Cutaneous Melanoma, With a Special Focus on Desmoplastic Melanoma., PMID:40505073
Adjuvant pembrolizumab following kidney cancer surgery A novel patient decision aid to facilitate shared decision-making., PMID:40503866
Managing severe dermatitis and grade 4 pneumonitis in a patient with non-small-cell lung cancer following pembrolizumab treatment: A case report., PMID:40503034
Metastatic renal cell carcinoma to the glans penis: A case report of rare presentation., PMID:40502920
Design and rationale of the phase II PANDORA trial: first line chemo-immunotherapy in advanced Merkel cell carcinoma., PMID:40501309
Phase 1 and 2 Clinical Studies of the STING Agonist Ulevostinag With and Without Pembrolizumab in Participants With Advanced or Metastatic Solid Tumors or Lymphomas., PMID:40499147
Impact of Tumor Response and Response Duration on Survival Among Participants Receiving Pembrolizumab Plus Chemotherapy as First-Line Therapy for Non-Small-Cell Lung Cancer., PMID:40498298
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Enfortumab vedotin induced interstitial lung disease: A first case report with pathological evidence from transbronchial lung cryobiopsy., PMID:40496844
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management., PMID:40496609
First-line pembrolizumab used concurrently with multi-agent chemotherapy and inhaled tranexamic acid (TXA) for management of stage III mixed trophoblastic tumor complicated by pulmonary hemorrhage., PMID:40496174
Response of EGFR-mutated pulmonary pleomorphic carcinoma to pembrolizumab., PMID:40491684
Pembrolizumab-induced toxic epidermal necrolysis with limited mucosal involvement., PMID:40491518
First-Line Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Retrospective, Multicenter, Real-World Study., PMID:40491285
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
A Phase 1/1B Trial of Pembrolizumab and Trametinib in Advanced NSCLC Enriched for KRAS Mutations., PMID:40486486
Second-line pembrolizumab for advanced HCC: Meta-analysis of the phase III KEYNOTE-240 and KEYNOTE-394 studies., PMID:40486134
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of belantamab mafodotin for multiple myeloma, asciminib for leukemia, osimertinib for lung cancer, amivantamab for lung cancer, and pembrolizumab for pleural mesothelioma in Japan., PMID:40481943
Two Cases of Enfortumab Vedotin-Induced Drug Eruption Diagnosed Based on the Distinctive Epidermal Mitotic Figures in Patients Receiving Combination Therapy With Pembrolizumab., PMID:40476598
Immune checkpoint inhibitor therapy in metastatic renal cell carcinoma: tumour response and immune-related renal vasculitis following cytoreductive nephrectomy., PMID:40474803
Assessing individual head and neck squamous cell carcinoma patient response to therapy through integration of functional and genomic data., PMID:40473660
Maintenance with niraparib in patients with stage III, stage IV, chemo-naïve recurrent or platinum-sensitive recurrent uterine serous carcinoma: study protocol for a phase II clinical trial., PMID:40473279
LEAP-008: Lenvatinib Plus Pembrolizumab for Metastatic NSCLC That Has Progressed After an Anti-PD-(L)1 Plus Platinum Chemotherapy., PMID:40473109
Tumor-stroma proportion on primary tumor as a prognostic biomarker in advanced ovarian cancer patients receiving chemo-immunotherapy as first-line therapy: analyses from the NeoPembrOV/GINECO phase II randomized trial., PMID:40472658
Risk factors for checkpoint inhibitor pneumonitis in lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis., PMID:40469302
Efficacy and safety of adjuvant TTFields plus pembrolizumab and temozolomide in newly diagnosed glioblastoma: A phase 2 study., PMID:40466642
Postimmunotherapy antiglial fibrillary acidic protein encephalomyelitis after adjuvant pembrolizumab for melanoma., PMID:40465327
Impact of medications on the efficacy of immune checkpoint inhibitors in patients with recurrent or metastatic head and neck cancer., PMID:40465127
[Network pharmacology and molecular docking explore mechanism of Croci Stigma in treating immune checkpoint inhibitor-associated myocarditis]., PMID:40461207
Enfortumab Vedotin Plus Pembrolizumab in Untreated Locally Advanced or Metastatic Urothelial Carcinoma: 2.5-Year Median Follow-Up of the Phase III EV-302/KEYNOTE-A39 Trial., PMID:40460988
New Treatment Approaches for Triple-Negative Breast Cancer., PMID:40460322
Case Report: Oral lichenoid mucositis in a patient with metastatic renal cell carcinoma undergoing treatment with pembrolizumab and axitinib., PMID:40458718
Cutaneous adverse effects of combination epidermal growth factor receptor inhibitor and immune checkpoint inhibitor cancer therapy., PMID:40455297
Erythema multiforme as a rare skin manifestation during pembrolizumab treatment: a case report and literature review., PMID:40454868
Remote Clinical Pharmacist Impact on Reducing Total Cost of Care in Enhancing Oncology Model-Enrolled Oncology Practices., PMID:40454696
Pembrolizumab Induced Aortitis in a Patient With Head and neck Cancer: A Case Report., PMID:40454445
Guidelines of Onkopedia: What is new? Esophageal Cancer., PMID:40451170
Enfortumab vedotin plus pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer (EV-302): patient-reported outcomes from an open-label, randomised, controlled, phase 3 study., PMID:40449498