The Monkeypox virus (MPXV) is an enveloped virus with a brick- or ovoid-shaped structure, containing a double-stranded DNA genome of approximately 197 kbp. It encodes around 200 genes essential for replication, transcription, and assembly.
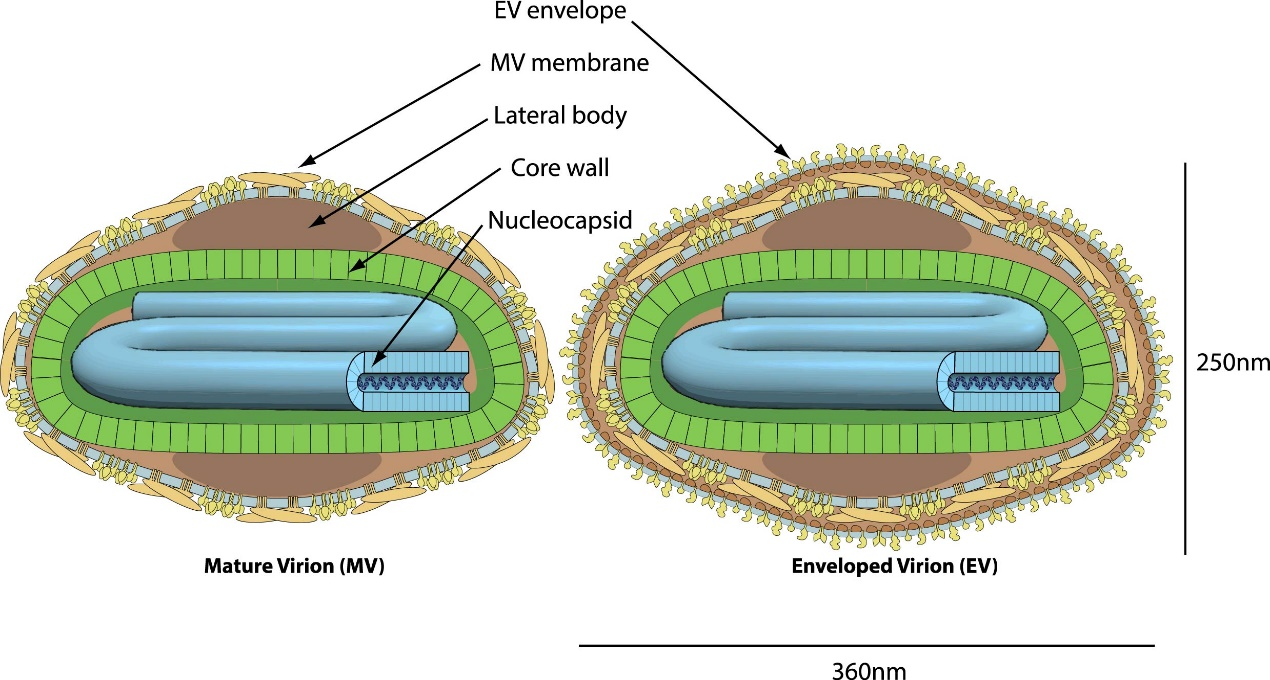
Fig 1. Structure of Mature and Enveloped Monkeypox Virions
Mpox, formerly known as monkeypox, was first identified in humans in the Democratic Republic of Congo (DRC) in 1970. Based on genomic studies, MPXV is classified into two main clades: Clade 1 (Central African or Congo Basin clade) and Clade 2 (West African clade). Clade 1, prevalent in Central Africa, has higher fatality rates ranging from 1% to 12%, primarily affecting children. Recently, a new sublineage within Clade 1, known as Clade 1b, has been identified. Clade 2, mainly found in West Africa, is less virulent, with a fatality rate of less than 0.1%. In 2022, during a global outbreak, a new lineage closely related to Clade 2, termed Clade 2b, emerged and spread widely through human-to-human transmission.
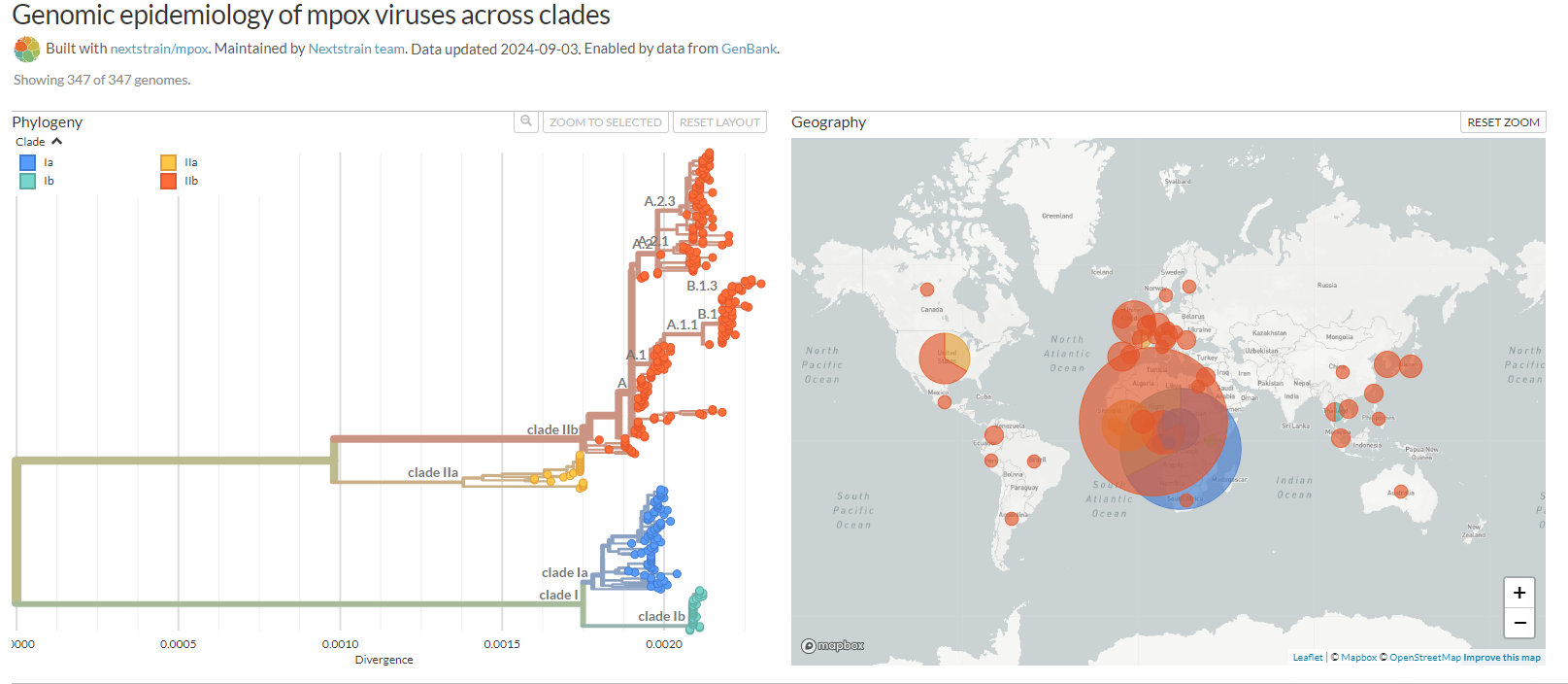
Fig 2. Genomic epidemiology of mpox viruses across clades.
Transmission of mpox primarily occurs from animals to humans through bites, scratches, or contact with infected animals, with human-to-human transmission possible via respiratory secretions, direct physical contact, vertical transmission, or contact with contaminated objects (fomites). Diagnostic methods include RT-PCR tests targeting conserved regions of genes such as the B6R and E9L. Vaccines such as JYNNEOS, a non-replicating live virus vaccine developed specifically for mpox, have been used effectively. Tecovirimat, an antiviral medication, is prescribed for severe cases or for those at risk of serious disease.
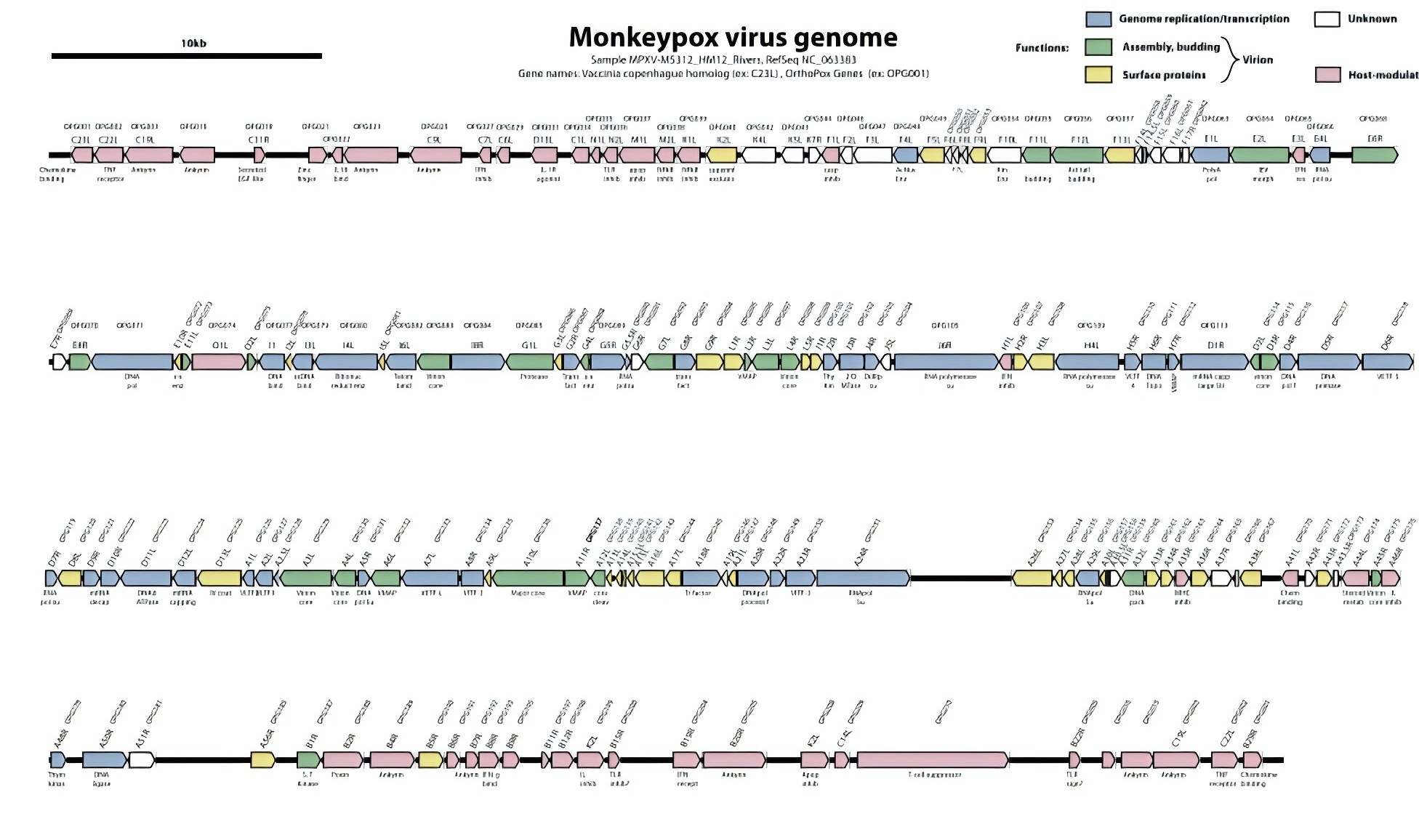
Fig 3. Monkeypox virus (MPXV) genome organization.
Several viral proteins play critical roles in mpox transmission, making them potential targets for vaccines and treatments. Key proteins include:
Several key proteins play critical roles in the Monkeypox virus (MPXV) transmission, making them potential targets for vaccines and treatments. A35R, a component of the extracellular enveloped virus (EEV), is homologous to the vaccinia virus (VACV) A33R protein and is crucial for cell-to-cell viral transmission. Vaccines targeting A33R have shown the ability to induce neutralizing antibodies, and A35R is similarly seen as a promising target for mpox vaccines, with some mRNA vaccines showing positive results. E8L, another important protein, binds to host cell surface chondroitin sulfate proteoglycans (CSPG) and gangliosides, potentially aiding in viral attachment. Predicted B-cell epitopes within E8L could be useful for vaccine development.
M1R is a highly conserved virion membrane protein that likely binds to host cell receptors, forming a complex that facilitates viral entry into the host cytoplasm. Similarly, A29L, homologous to VACV A27L, is a vesicle membrane protein on the surface of intracellular mature virions (IMV), playing a key role in viral attachment and serving as a target for neutralizing antibodies. B6R, which encodes a 40 kDa type I transmembrane protein, functions as an extracellular envelope protein and serves as a specific target for mpox diagnostics due to its homology with the VACV B5R gene.
Other proteins like D14L act defensively for the virus, serving as a monkeypox inhibitor of complement enzymes (MOPICE) by inhibiting complement activation pathways, while H3L facilitates virion attachment to host cells by binding to heparan sulfate and inducing cellular damage through upregulation of inflammatory responses, such as IL1A expression. Together, these proteins represent vital components of MPXV's infectivity and potential targets for therapeutic intervention.
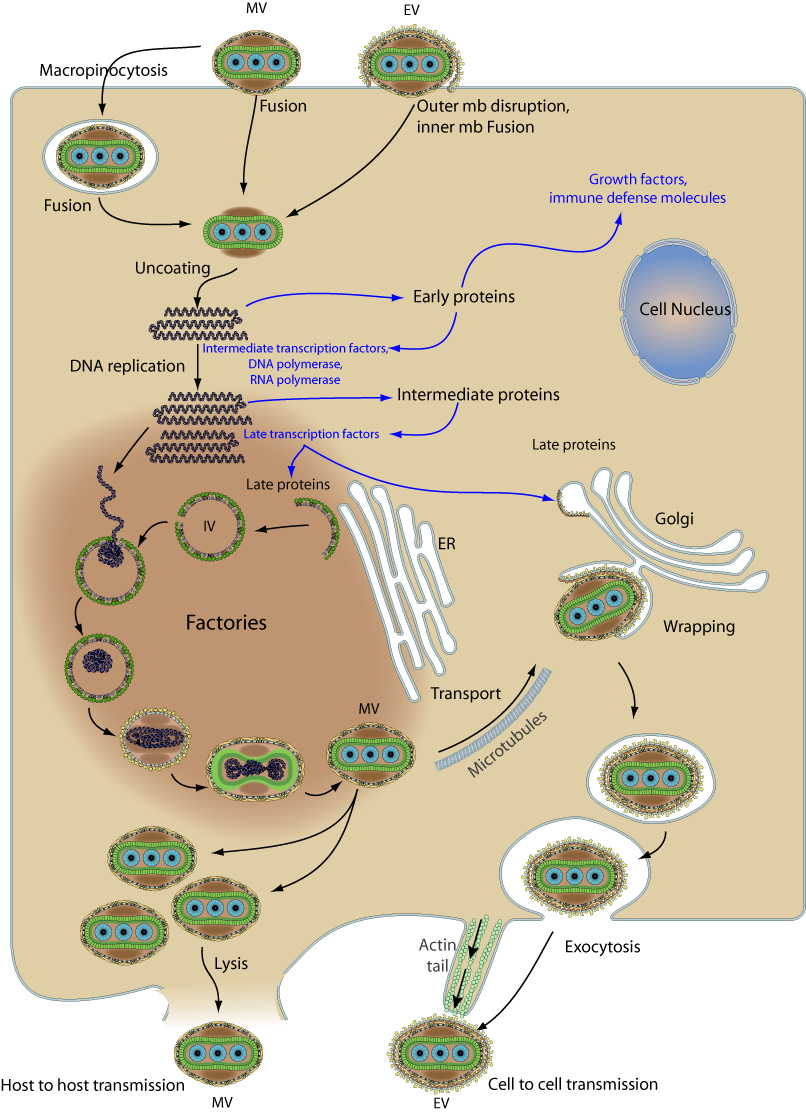
Fig 4.Replication and life cycle of the Monkeypox virus (MPXV).
Ongoing research is dedicated to advancing our understanding of the key viral proteins involved in the transmission, replication, and immune evasion of the Monkeypox virus (MPXV). By focusing on these proteins, scientists are working to enhance diagnostic tools, enabling quicker and more accurate detection of the virus in affected populations. In parallel, efforts are being made to develop more effective treatments and vaccines that specifically target these viral components, such as A35R, E8L, M1R, and B6R, which play crucial roles in the virus's lifecycle and interaction with host cells.
In addition to improving diagnostics, research is also exploring therapeutic approaches, including antiviral medications like Tecovirimat and innovative vaccine strategies that could offer broader and longer-lasting protection. The goal is to not only manage current outbreaks more effectively but also to provide preventive measures that can reduce the global impact of mpox in the future. By focusing on these critical viral proteins and understanding their functions, researchers aim to create targeted interventions that could significantly improve global control and prevention efforts against mpox, ultimately minimizing its public health threat.
More information: There is a current outbreak of mpox/monkeypox (2024). WHO. https://www.who.int/home/search-results?indexCatalogue=genericsearchindex1&searchQuery=monkeypox&wordsMode=AnyWord.
Information by WHO
