A study published in Science Translational Medicine explores the development and efficacy of two potent neutralizing antibodies targeting the fusion glycoprotein (F protein) of Nipah virus (NiV). This research, conducted by Mapp Biopharmaceuticals in collaboration with multiple research institutions, aims to develop more effective antibody-based therapies against this deadly pathogen.
Title:Therapeutic administration of a cross-reactive mAb targeting the fusion glycoprotein of Nipah virus protects nonhuman primates
Science Translational Medicine· 3 Apr 2024 Vol 16, Issue 741· DOI:10.1126/scitranslmed.adl2055

Background on Nipah Virus and Antibodies
Nipah virus is a highly lethal zoonotic pathogen responsible for severe respiratory disease and encephalitis, particularly in South and Southeast Asia. Despite its significant threat, there are currently no approved vaccines or specific treatments for Nipah virus. An earlier developed human monoclonal antibody, m102.4(RVV08002), has been tested in a phase 1 clinical trial and used under compassionate use for post-exposure prophylaxis. However, m102.4's protective efficacy has been limited in certain cases, highlighting the need for more effective antibodies.
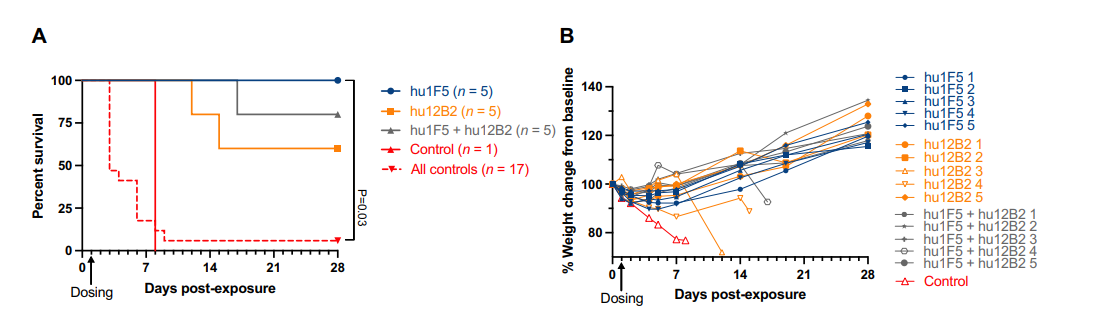
Fig. 1. Anti–F protein mAbs are efficacious in hamsters. (A)
Key Findings of the Study
The research team compared two neutralizing antibodies: hu1F5 and hu12B2, both targeting the F protein of Nipah virus. In hamster models, hu1F5 demonstrated significantly higher efficacy in protecting animals from Nipah virus infection. Consequently, hu1F5 was selected for further comparison with m102.4 in African green monkey (AGMs) models.
The results were striking: all AGMs treated with hu1F5 survived the infection, whereas only one of the AGMs treated with m102.4 survived. This result underscores the superior protection offered by hu1F5 against Nipah virus.
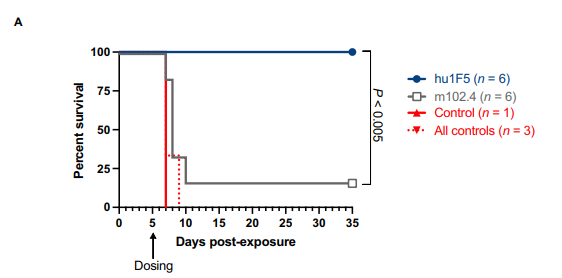
Fig 2.illustrates the survival rates of AGMs treated with hu1F5 compared to m102.4, clearly showing hu1F5's enhanced protective effect.
Therapeutic Potential of hu1F5
Therapeutic Potential of hu1F5
The hu1F5 antibody works by targeting the prefusion conformation of the F protein, preventing it from transitioning to the postfusion state required for viral infection. This mechanism of action enables hu1F5 to neutralize the virus effectively, both in vitro and in vivo. To enhance its clinical potential, the research team introduced mutations to extend the antibody's half-life, making hu1F5 a strong candidate for use in both prophylactic and therapeutic settings.
Fig. 3. Plasma viral loads were reduced after hu1F5 and m102.4 mAb treatment.
The study’s findings support hu1F5 as a promising candidate for further development as a post-exposure prophylactic and therapeutic option against Nipah virus. The research team plans to advance hu1F5 into phase 1 clinical trials to assess its safety and efficacy in humans. If successful, hu1F5 could become a critical tool in the fight against Nipah virus outbreaks.
Fig 4. show the protective efficacy and viral load changes in AGMs treated with reduced doses of hu1F5, demonstrating the antibody's potential clinical application at different dosages.
This study reveals the significant potential of neutralizing antibodies, particularly hu1F5, in combating Nipah virus. In the absence of other effective treatment options, hu1F5 could provide a new pathway for preventing and treating this deadly disease. Upcoming clinical trials will be crucial in determining its effectiveness in humans and may offer new hope for controlling future Nipah virus outbreaks.
AntibodySystem SAS is committed to providing high-quality reagents to support the research and diagnostics community in their study of Nipah virus. Our catalog includes monoclonal antibodies targeting Nipah virus, m102.4 and m102.3 (RVV08002 and RVV08001). AntibodySystem SAS’s engineering platform allows us to offer these antibodies in various isotype and species formats, including rabbit, human, and mouse. If you have any inquiries or need further information, please feel free to reach out to us.
Get in touch:support@antibodysystem.com
More information: Larry Zeitlin et al, Therapeutic administration of a cross-reactive mAb targeting the fusion glycoprotein of Nipah virus protects nonhuman primates, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adl2055. www.science.org/doi/10.1126/scitranslmed.adl2055
Journal information: Science Translational Medicine
