When starting recombinant protein expression, many beginners quickly encounter the same fundamental question:
“Which expression system should I choose for my protein?”
E. coli, yeast, insect cells, mammalian cells—there seems to be no shortage of options.
What truly causes confusion, however, is not what systems exist, but rather why the same gene can yield dramatically different results when expressed in different systems.
The answer is actually quite straightforward: different expression systems differ greatly in their ability to properly “handle” a protein.
I. A Core Concept to Understand First
The essential differences between expression systems go far beyond simply “which host cell is used” or “whether the protein can be expressed at all.”
At their core, these systems differ in whether they can produce the protein correctly.
Here, “correctly” refers to several critical aspects:
- Proper protein folding
- Appropriate post-translational modifications (PTMs)
- Retention of native biological activity
Not every expression system is capable of meeting all these requirements.
II. Overview of Major Expression Systems
1. Escherichia coli Expression System
For many researchers, E. coli is the first expression system they encounter. It is a prokaryotic system that is highly efficient, but relatively poor at caring for complex proteins. Its advantages are obvious: rapid growth, simple operation, low cost, and often very high expression yields. As a result, E. coli is well suited for proteins of prokaryotic origin or proteins with relatively simple structures that do not rely on post-translational modifications.
However, these strengths also define its limitations. E. coli lacks the folding machinery and post-translational modification pathways found in eukaryotic cells. When used to express eukaryotic proteins—especially at high expression levels—misfolding and inclusion body formation are common. In practice, this often manifests as strong Western blot signals but little to no biological activity, or poor protein solubility. Importantly, this is not necessarily due to experimental error, but rather reflects intrinsic limitations of the system itself.
2. Yeast Expression System
When protein folding becomes more demanding, yeast expression systems are often considered an upgrade. Yeast are lower eukaryotes that possess basic eukaryotic folding mechanisms, including disulfide bond formation and simple glycosylation, while still maintaining relatively fast growth and moderate cost.
For proteins that require basic post-translational modifications but are not highly sensitive to glycosylation patterns, yeast can offer an attractive balance between performance and cost. However, it is important to note that yeast glycosylation differs fundamentally from that of mammalian cells. Yeast predominantly produce high-mannose-type glycans, which may affect protein stability, serum half-life, or even biological function in certain applications.
Thus, the key question when using yeast is often not whether the protein can be expressed, but whether the downstream application can tolerate these glycosylation differences.
3. Insect Cell–Baculovirus Expression System
For projects seeking higher success rates without the full cost and complexity of mammalian cell culture, the insect cell–baculovirus system is often a highly reliable option. This system offers protein folding, solubility, and post-translational modification capabilities that closely approximate those of mammalian cells.
In practice, many proteins that repeatedly fail in E. coli can be successfully expressed in insect cells. This system is particularly well suited for secreted proteins, structurally complex proteins, and extracellular domains of membrane proteins. While insect cell expression involves longer timelines and higher costs than bacterial or yeast systems, its overall robustness makes it a widely used and trusted platform in academic research.
4. Mammalian Cell Expression System
When protein function is highly dependent on native conformation and precise post-translational modifications, the range of viable options becomes limited. Mammalian expression systems are considered the gold standard because they most closely replicate the protein’s natural state in the human body.
Complex N-linked glycosylation, subtle conformational features, and protein–protein interaction interfaces are all best reproduced in mammalian cells. Consequently, mammalian expression is often indispensable for therapeutic antibodies, receptor proteins, critical cytokines, and any protein where biological activity must be preserved with high fidelity.
These advantages, however, come with practical trade-offs: longer culture times, higher operational complexity, and significantly increased cost.
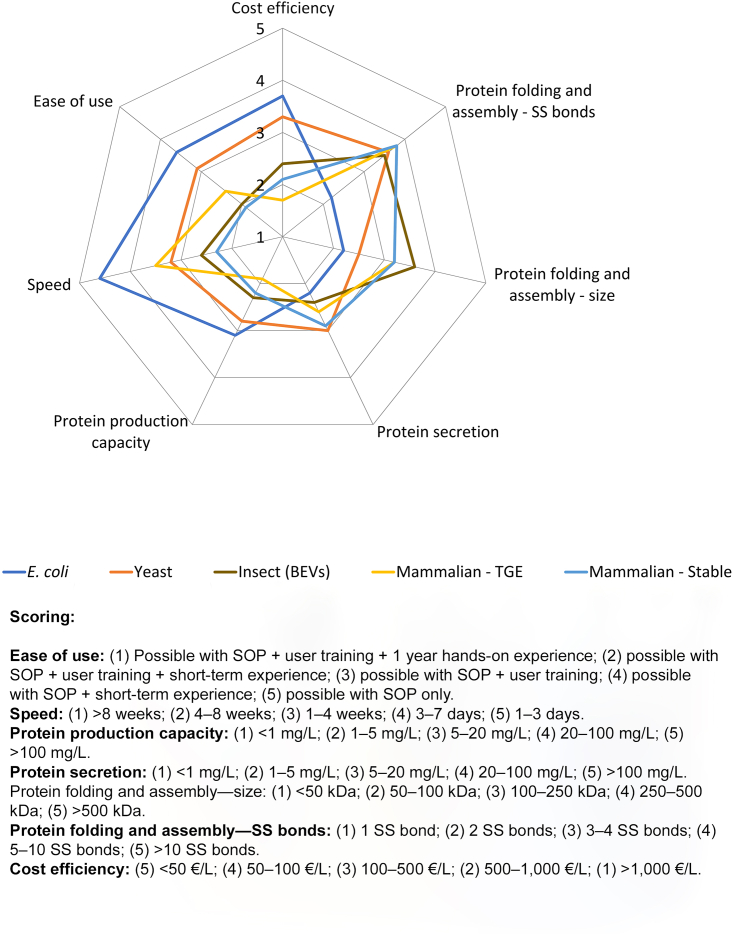
Comparative overview of the characteristics associated with the major gene expression systems(STAR Protoc. 2023 Oct 31;4(4):102572.)
Practical Tips for Beginners
For newcomers to recombinant protein expression, a simple and practical decision-making strategy can be extremely helpful. If the protein originates from a prokaryote and has a simple structure, E. coli is often the most efficient starting point. For proteins derived from eukaryotic organisms—especially secreted or membrane proteins—eukaryotic expression systems should be considered early on.
As protein size increases, the number of disulfide bonds grows, or functional dependence on glycosylation becomes evident, the risks associated with prokaryotic expression increase dramatically.
In practical terms:
· Low-cost, high-yield expression of structurally simple proteins → E. coli
· Proteins requiring basic modifications but insensitive to glycan structure → Yeast
· Structurally complex proteins with higher success-rate requirements → Insect cells
· Proteins where native structure and function are non-negotiable → Mammalian cells
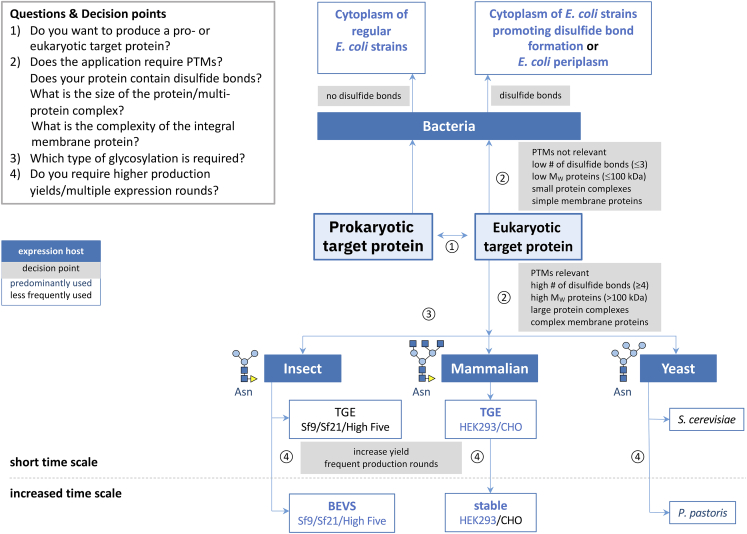
Decision scheme for gene expression system selection
(STAR Protoc. 2023 Oct 31;4(4):102572.)
AntibodySystem provides Active Protein and Recombinant Protein products, delivering more tools and solutions for research.
Active Protein
|
Catalog No. |
Product name |
|
AHB39502 |
Recombinant Human CD335/NCR1/NKp46 Protein, C-His (Active) |
|
AHC06701 |
Recombinant Human CSF2/GM-CSF Protein, C-His (Active) |
|
AHC13501 |
Recombinant Human IL4 Protein, C-His (Active) |
|
AHC14301 |
Recombinant Human SERPING1 Protein, C-His (Active) |
|
AHC30501 |
Recombinant Human CD271/NGFR Protein, C-hFc-His (Active) |
|
AHC35001 |
Recombinant Human CD16a/FCGR3A (F176) Protein, C-His (Active) |
|
AHC94101 |
Recombinant Human GZMA/Granzyme A Protein, C-10*His (Active) |
|
AHD69401 |
Recombinant Human PTX3 Protein, C-His (Active) |
|
AHE01701 |
Recombinant Human CD50/ICAM3 Protein, C-hFc-His (Active) |
|
AHE79001 |
Recombinant Human CD239/BCAM Protein, C-His (Active) |
|
AHJ64101 |
Recombinant Human ESM1 Protein, C-10*His (Active) |
|
AHJ73201 |
Recombinant Human DKK3 Protein, C-His (Active) |
|
AHK15501 |
Recombinant Human SEMA4C Protein, C-hFc (Active) |
|
AMB89901 |
Recombinant Mouse C3/Complement C3 Protein, C-His (Active) |
|
AMB90001 |
Recombinant Mouse C5/Complement C5 Protein, No tag (Active) |
|
AMC42101 |
Recombinant Mouse CSF1/M-CSF Protein, C-His (Active) |
|
AMD29101 |
Recombinant Mouse LBP Protein, C-His (Active) |
|
AME70701 |
Recombinant Mouse Flt3 ligand/FLT3LG Protein, C-hFc-His (Active) |
|
APC06701 |
Recombinant Pig CSF2/GM-CSF Protein, C-His (Active) |
|
ARB95301 |
Recombinant Rat IFNB1/IFN-beta Protein, C-His (Active) |
Recombinant Protein
|
Catalog No. |
Product name |
|
EVV00348 |
Recombinant SARS-CoV-2 RBD (XDV.1) Protein, C-Fc |
|
EVV03501 |
Recombinant RABV G/Glycoprotein, C-His |
|
EVV03502 |
Recombinant Rabies virus/RABV G/Glycoprotein Protein, C-10*His |
|
EVV12704 |
Recombinant MPXV H3L Protein, C-His |
|
EVV12703 |
Recombinant Monkeypox virus (MPXV) H3L Protein, C-Fc |
|
EVV18201 |
Recombinant Langya virus/LayV F/Fusion Protein, C-His |
|
YHA19001 |
Recombinant Human UBE2C Protein, N-His |
|
YHA19301 |
Recombinant Human SCD Protein, N-His |
|
YHA17301 |
Recombinant Human PIR Protein, N-His |
|
YHA16901 |
Recombinant Human FCN1 Protein, N-His |
|
YHA11501 |
Recombinant Human DRP1/DNM1L Protein, N-His |
|
YHA05301 |
Recombinant Human CD284/TLR4 Protein, N-His |
|
EVV02801 |
Recombinant HRSV-A2 Pre-F/Fusion glycoprotein F0 Protein, C-His-Strep |
|
EVV03001 |
Recombinant HBV S/L-HBsAg/L glycoprotein Protein, C-S Tag |
|
EVV15201 |
Recombinant EBV/HHV4 BLLF1/MA/GP350 Protein, C-His |
|
EVV09701 |
Recombinant CHIKV Spike glycoprotein E2 Protein, C-Fc |
|
EVV16002 |
Recombinant ASFV p54/pE183L Protein, C-His |
