A Step-by-Step Tutorial to Visualize Protein Localization in Cells
If you are:
·New to immunofluorescence (IF)
·Impressed by beautiful IF images but unsure how they are generated
·Feeling nervous about performing your first IF experiment
This guide is written for you.
Without assuming prior experience, this tutorial walks you through a practical, reliable, and reproducible immunofluorescence workflow, from experimental setup to image acquisition.
1. What Is Immunofluorescence and Why Do We Use It?
Immunofluorescence (IF) is a technique that uses fluorescently labeled antibodies to visualize the subcellular localization of a specific protein within cells.
In simple terms:
IF tells you where a protein is located inside a cell.
Conceptual analogy (for beginners)
·A cell is like a dark room
·Proteins are people inside the room
·Antibodies act as highly specific “recognition tools”
·Fluorophores function as small light sources
When viewed under a fluorescence microscope, the protein appears exactly where the fluorescence signal is detected.
What questions can IF answer?
·Is the protein located in the nucleus, cytoplasm, or plasma membrane?
·Do two proteins co-localize within the same cellular compartment?
·Does protein localization change after drug treatment or stimulation?
These spatial questions cannot be answered by Western blotting, which only provides presence or absence of a protein.
2. Experimental Preparation: What Do You Need?
Key principle:
You do not need to understand everything at once—only what each component is used for.
Cell Culture Materials
|
Item |
Description |
Key Notes |
|
Coverslips |
Thin glass surfaces |
Cells must adhere firmly |
|
24-well plate |
Holds coverslips |
Beginner-friendly format |
|
Cultured cells |
Experimental subject |
Poor cell condition leads to failure |
Three Essential Reagents You Must Understand
1) Fixative (4% Paraformaldehyde, PFA)
·Purpose: Preserves cell morphology and protein localization
·Without fixation: Proteins may diffuse or redistribute
Think of fixation as taking a permanent snapshot of the cell.
2) Permeabilization Reagent (Triton X-100)
·Purpose: Creates pores in the cell membrane
·Allows antibodies to access intracellular targets
·Mandatory for nuclear proteins
3) Blocking Solution (BSA or Normal Serum)
·Purpose: Prevents nonspecific antibody binding
·Poor blocking leads to high background fluorescence
Antibodies (The Core of IF)
|
Reagent |
Function |
|
Primary antibody |
Specifically binds the target protein |
|
Fluorescent secondary antibody |
Binds primary antibody and emits fluorescence |
|
DAPI |
Blue fluorescent dye for nuclear staining |
3. Complete Experimental Workflow (Day-by-Day)
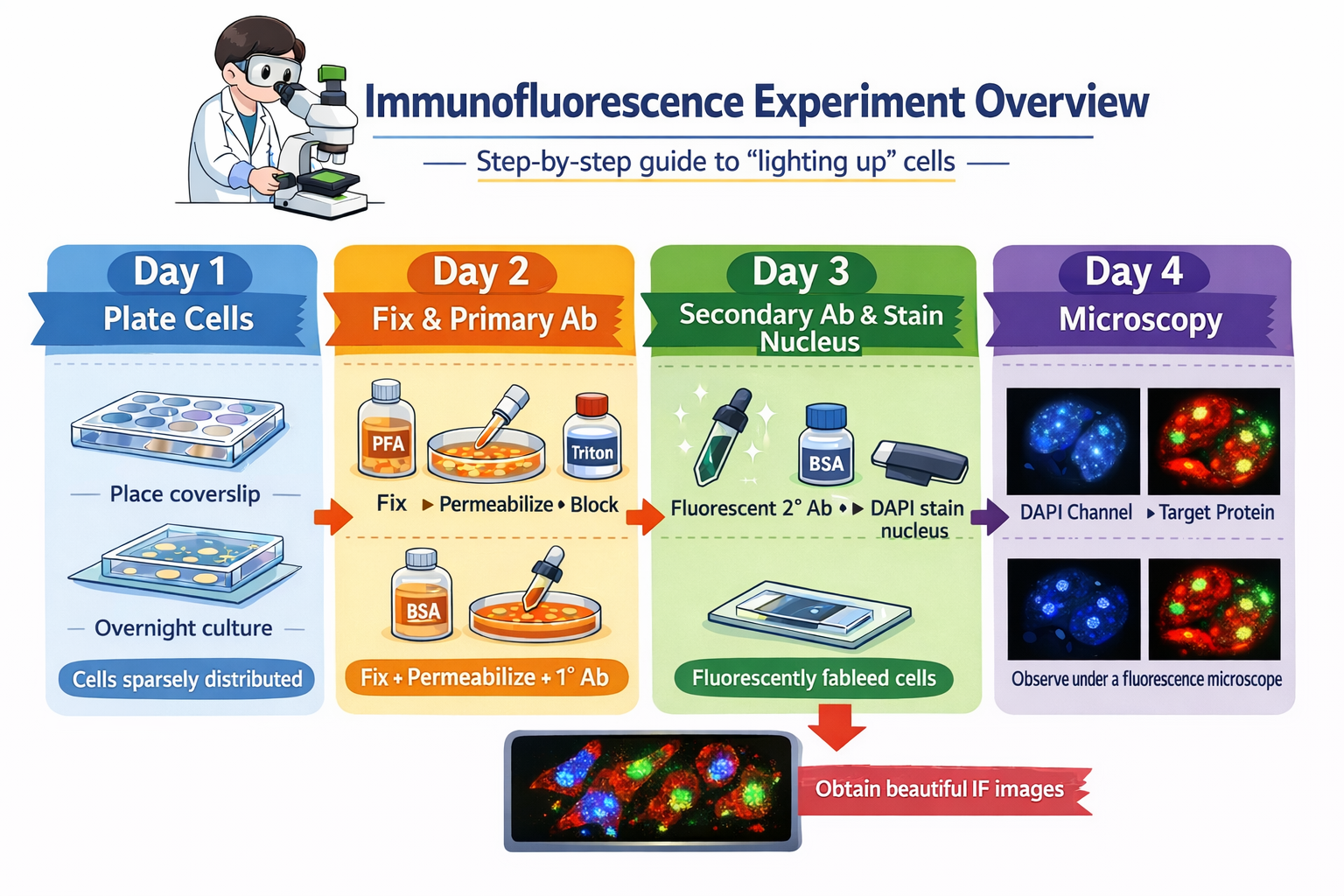
Day 1: Cell Seeding
Goal: Achieve evenly distributed, well-adhered cells
This step determines ~40% of experimental success.
Key principles
·Cells should be sparsely distributed
·Avoid over-confluence
·Even distribution is critical for image clarity
Procedure
1. Place a sterile coverslip at the center of each well in a 24-well plate.
2. Prepare a single-cell suspension using standard passaging procedures.
3. Adjust cell density to 1–5 × 10⁴ cells/mL in complete culture medium.
4. Gently add 1 mL of cell suspension to each well along the wall.
5. Gently swirl the plate in a cross pattern to distribute cells evenly.
6. Incubate at 37 °C, 5% CO₂ overnight (16–24 hours).
Day 2: Fixation, Permeabilization, Blocking, and Primary Antibody Incubation
This is the most critical day of the experiment
Step-by-step
1. Wash cells
· Aspirate medium carefully.
· Wash 3× with cold PBS.
· Use gentle pipetting to avoid cell detachment.
2. Fixation
·Add 1 mL of 4% PFA per well.
·Incubate at room temperature for 20 minutes.
·Wash 3× with PBS.
3. Permeabilization
·Add 0.1–0.5% Triton X-100.
·Incubate at 4 °C for 10 minutes.
·Wash 5× with PBS.
Required for nuclear proteins.
Optional or reduced for extracellular epitopes.
4. Blocking
·Incubate with 3–5% BSA for 30 minutes at room temperature.
·Do not wash after blocking.
5. Primary antibody incubation
·Dilute primary antibody in blocking buffer (e.g., 1:200).
·Add 200–300 µL per well.
·Incubate overnight at 4 °C.
At this point, the core immunofluorescence staining is complete.
Day 3: Secondary Antibody Staining, Nuclear Counterstain, and Mounting
Protect samples from light from this point onward
Procedure
1. Wash primary antibody
·Wash 4× with PBS, 5 minutes each.
2. Secondary antibody incubation
·Dilute fluorescent secondary antibody (e.g., 1:500).
·Incubate for 1 hour at room temperature, protected from light.
3. Wash
·Wash 3× with PBS, 5 minutes each.
·DAPI staining
4. Dilute DAPI (typically 1:1000).
·Incubate for 3–5 minutes.
·Wash 2× with PBS.
5. Mounting
·Place 10–20 µL of antifade mounting medium on a glass slide.
·Mount coverslip cell-side down.
·Avoid air bubbles.
·Allow to cure at room temperature in the dark for ≥4 hours.
Day 4: Fluorescence Microscopy and Image Acquisition
Recommended observation sequence
1. Locate cells using bright-field illumination.
2. Switch to DAPI channel (405 nm).
3. Observe target protein using appropriate fluorescence channel:
·FITC / Alexa Fluor 488 → 488 nm
·TRITC / Alexa Fluor 568 → 561 nm
Imaging tips
·Adjust exposure to avoid saturation.
·Keep imaging parameters consistent across samples.
·Acquire single-channel and merged images.
Images can be analyzed using ImageJ/Fiji for intensity or colocalization analysis.
Congratulations!
If you have completed all steps successfully, you have performed your first immunofluorescence experiment.
Immunofluorescence is not about "perfect hands", it is about understanding the logic behind each step.
Practical recommendations
·Perform the first experiment with guidance from an experienced colleague
·Always include negative controls (no primary antibody)
·Optimization, not failure, explains most initial problems
|
Catalog |
Product Name |
|
RGK23942 |
Anti-DNA Antibody(F2-3) |
|
RGK24020 |
Anti-dsDNA Antibody(3E10#) |
|
RGK24303 |
Anti-6,4DNA/6-4PPs Antibody (64M-2) |
|
FHK18510 |
Anti-Human CD8 Antibody(G10-1) |
|
RGK08501 |
Anti-Flag Tag (DYKDDDDK) Antibody (M2) |
|
RGK25601 |
Anti-SSEA4 Antibody (MC813-70) |
|
RGK26002 |
Anti-polyglutamine/polyQ Antibody (1C2) |
|
FHD10840 |
Anti-Human CD19 Antibody (FMC63) |
|
RHC59303 |
Anti-K63-linked Polyubiquitin Antibody (SAA0330) |
|
RVV03302 |
Anti-Influenza A virus NP/Nucleoprotein Antibody (SAA0411) |
|
RGK11203 |
Anti-Ganglioside GM3/NeuGcGM3 Antibody (SAA0555) |
|
RGK32701 |
Anti-GXM/glucuronoxylomannan Antibody (SAA0561) |
