Colon cancer (COC) is a malignant tumor with persistently high global incidence and mortality rates. Despite advancements in surgery, chemotherapy, and targeted therapies, issues of tumor metastasis and chemoresistance remain severe, leading to limited improvement in patient prognosis. In recent years, a novel, iron-dependent form of regulated cell death—ferroptosis—has garnered significant attention for its potential in cancer therapy. It primarily involves iron-mediated Fenton reactions generating reactive oxygen species (ROS), depletion of glutathione (GSH), inhibition of the key antioxidant enzyme GPX4, and the consequent lethal accumulation of lipid peroxides (LPO). However, a major challenge lies in inducing ferroptosis efficiently and specifically at tumor sites while avoiding off-target toxicity to normal tissues.

The study titled "Self-assembled phenylboronic acid nanomedicine targets sialic acid to synergistically activate ferroptosis via RRM1 suppression and GPX4 Inhibition for precision colon cancer therapy", published in Journal of Nanobiotechnology, aimed to develop a novel actively targeted nanomedicine to overcome the limitations of traditional ferroptosis inducers. The research team chemically conjugated artesunate (ART, an antimalarial drug known for its ferroptosis-inducing potential) with alanine (Ala, to improve water solubility) and phenylboronic acid (PBA, as a targeting moiety) to synthesize the ART-Ala-PBA (AAP) derivative. This derivative can self-assemble in aqueous solution to form carrier-free nanoparticles (AAP NPs). The core design rationale is to leverage the specific, reversible covalent binding between PBA and sialic acid (SA), which is overexpressed on the surface of many cancer cells, to achieve active targeted drug delivery, thereby enriching the drug at the tumor site and efficiently inducing ferroptosis.
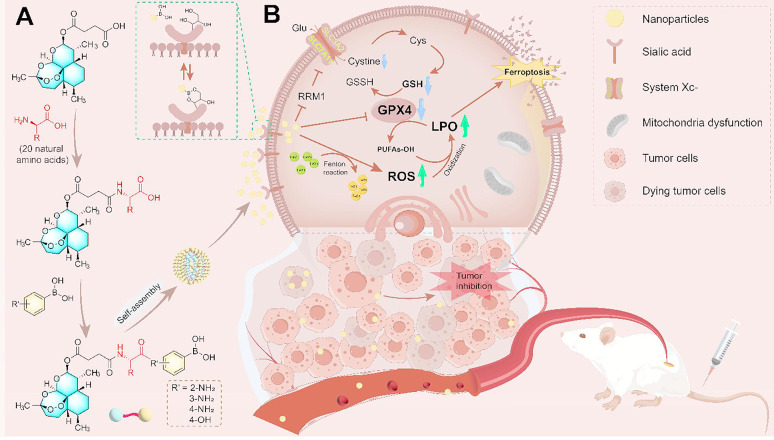
Figure 1. Schematic representation of artesunate derivative NPs with phenylboronic acid that target sialic acid to induce ferroptosis in colon cancer cells
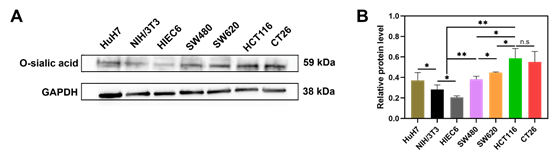
To validate the feasibility of the targeting strategy, this study first needed to confirm the overexpression of SA on the surface of colon cancer cells. At this crucial step, the researchers utilized AntibodySystem's Anti-Neu5Ac/O-sialic acid/Polysialic acid Antibody (Ab735) (Catalog No. RGK29702) for Western Blot analysis to detect SA expression levels in colon cancer cell lines. The experimental results showed that SA expression was significantly higher in all tested tumor cells compared to normal cells, with CT26 and HCT116 cells exhibiting the highest expression. This experiment provided critical evidence for the subsequent use of PBA for active targeting, confirming the feasibility of SA as a target in colon cancer, forming the experimental foundation for the targeting strategy design in this study.
The main content and in-depth interpretation of the research are as follows: First, regarding physicochemical characterization, the successfully prepared AAP NPs exhibited uniform size, negative surface charge, and rapid drug release under the acidic tumor microenvironment, which is conducive to drug accumulation and efficient release within tumor tissues. Cellular uptake experiments further confirmed their targeting capability: compared to PBA-free ART-Ala NPs, AAP NPs showed significantly higher internalization efficiency in CT26 and HCT116 cells, and this process could be competitively inhibited by free PBA. This indicates that the binding between PBA and cell surface SA is the primary mechanism mediating the specific uptake of AAP NPs.
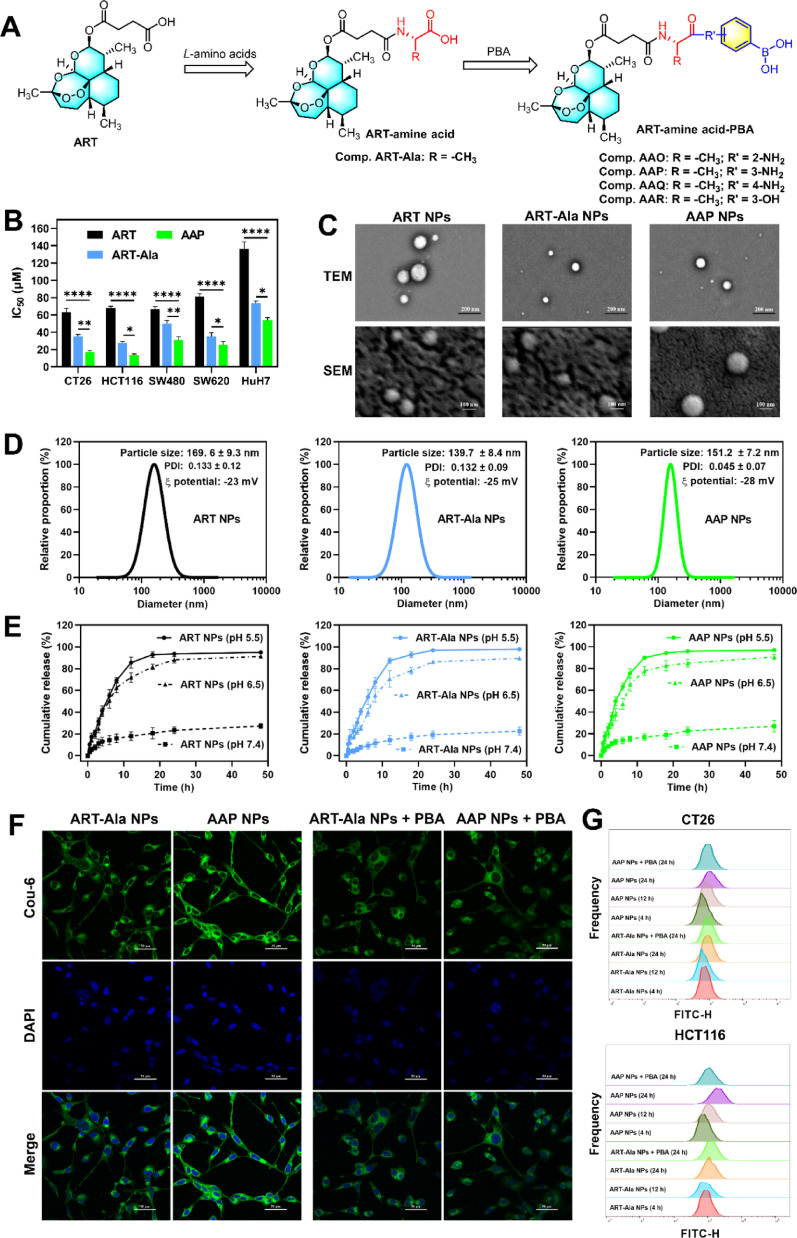
Figure 2. Cell viability of artesunate derivatives in vitro; physicochemical characterization of ART NPs, ART-Ala NPs, and AAP NPs; and assessment of their cellular uptake
Regarding antitumor efficacy and mechanism exploration, in vitro experiments demonstrated that AAP NPs exhibited potent inhibitory effects on the proliferation, clonogenic survival, migration, and invasion of multiple colon cancer cell lines (especially CT26 and HCT116 with high SA expression), outperforming the parent ART drug and its simple derivative ART-Ala NPs.
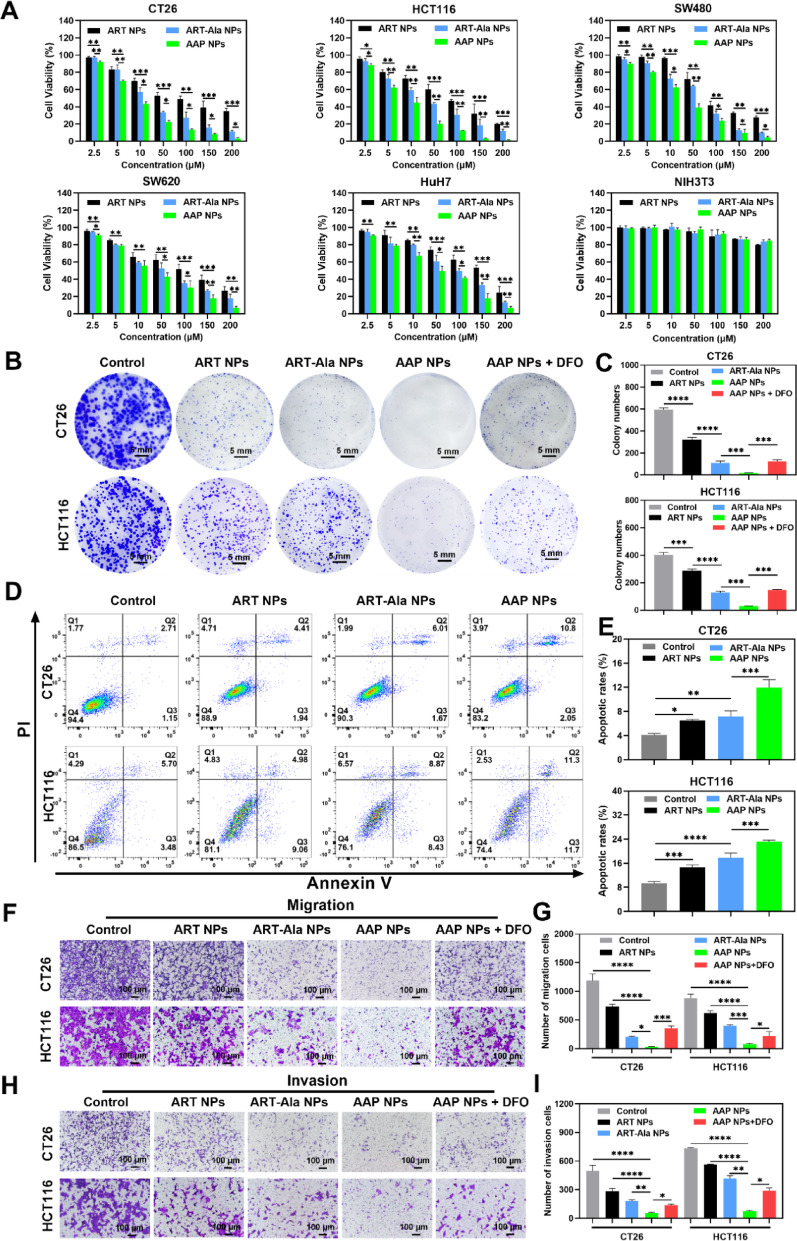
Figure 3. In vitro antitumor efficacy of the NPs
Importantly, ferroptosis inhibitors (DFO and Fer-1) could significantly reverse the cell death caused by AAP NPs, suggesting ferroptosis as the primary lethal mechanism. In-depth mechanistic studies revealed that AAP NPs effectively increased intracellular ROS levels, depleted GSH, inhibited GPX4 activity and protein expression, while causing significant accumulation of LPO and its end product MDA, and inducing mitochondrial membrane potential loss and morphological damage. These are classic hallmarks of ferroptosis.
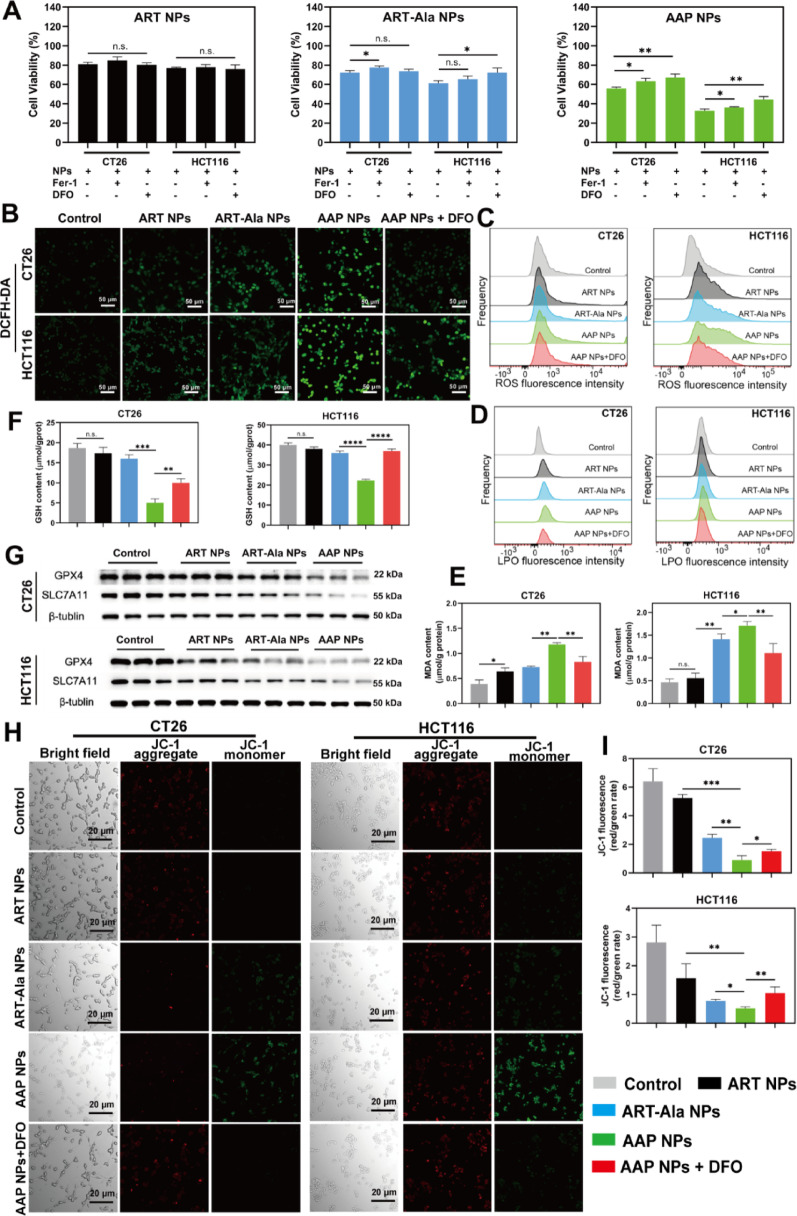
Figure 4. AAP NPs trigger ferroptosis in tumor cells in vitro
The most significant innovation of this study lies in the discovery of a novel target through deep data-independent acquisition (DIA) proteomics: the ribonucleotide reductase regulatory subunit M1 (RRM1). Proteomic analysis revealed that AAP NPs specifically downregulated RRM1 expression. RRM1 is a key enzyme for DNA synthesis and repair, and previous studies have suggested its association with tumor progression and drug resistance. Combined with bioinformatic analysis, this study found that RRM1 expression positively correlated with key ferroptosis regulators like SLC7A11, and that RRM1 knockdown could promote ferroptosis by affecting the p53-p21-GPX4 signaling axis. Therefore, AAP NPs may generate a synergistic effect by simultaneously inhibiting the classic GPX4/SLC7A11 defense axis and the emerging RRM1-related pathway, thereby "attacking from two fronts" to amplify ferroptosis signaling. This differs from the mechanism of traditional ART, which primarily affects amino acid metabolism, and RNA sequencing also suggested that AAP NPs uniquely modulated autophagy-related processes. This multimodal mechanism of action may be the source of its high efficacy.
In vivo experiments strongly supported the above findings. Near-infrared imaging showed that DiR-labeled AAP NPs accumulated and were retained in the tumor sites of CT26 tumor-bearing mice to a significantly greater extent and for a longer duration than non-targeted ART-Ala NPs. In therapeutic experiments, AAP NPs demonstrated powerful tumor growth inhibition (with a Tumor Growth Inhibition, TGI, of 73.5%), significantly outperforming control NPs, and even surpassing the clinical chemotherapeutic drug 5-FU and the classic ferroptosis inducer RSL3, while also significantly prolonging mouse survival. Analysis of tumor tissues again confirmed that in the AAP NPs treatment group, GPX4/SLC7A11 protein expression was downregulated, ROS and MDA levels were elevated, and GSH was depleted, validating that it also functions by inducing ferroptosis in vivo.
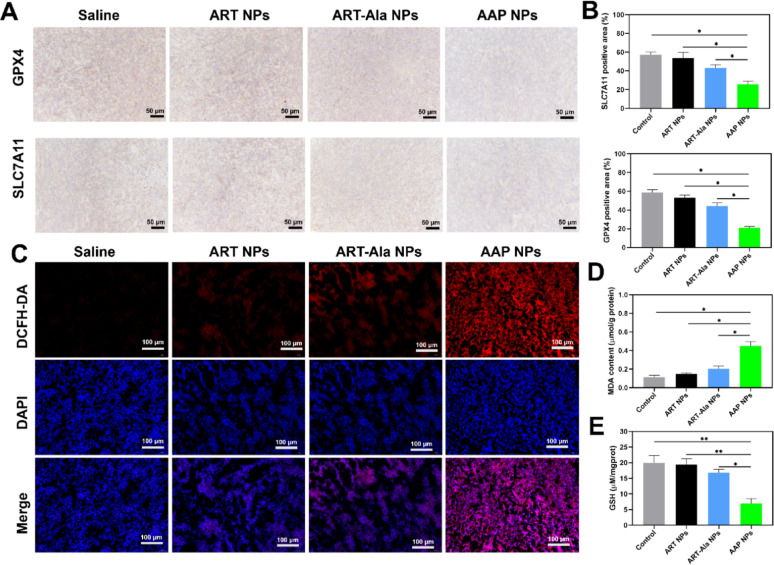
Figure 5. AAP NPs trigger ferroptosis in vivo
Certainly, this study also has some limitations and challenges, as discussed in the "Limitations and challenges in clinical translation" section of the paper. These include differences between animal models and the human tumor microenvironment, the yet undefined precise downstream molecular pathways of RRM1, potential off-target risks due to the presence of SA in some normal tissues, and translational medicine issues such as large-scale production, stability, and pharmacokinetics of the nanomedicine, all of which require further exploration in future research.
In summary, this study successfully developed a novel, self-assembled SA-targeted nanomedicine, AAP NPs. It achieves efficient accumulation in tumors through PBA-mediated active targeting and, for the first time, induces potent ferroptosis by synergistically inhibiting the dual pathways of RRM1 and GPX4/SLC7A11, while also modulating autophagy. This research provides a new strategy to overcome chemoresistance in colon cancer and offers a translatable paradigm for the design of precision nanomedicines based on TACA targets. The TACA antibodies from AntibodySystem played a crucial supporting role in target validation in this study.
TACA antibodies are a class of specific antibodies targeting carbohydrate antigens generated by abnormal glycosylation modifications (such as sialylation, fucosylation, etc.) on the surface of tumor cells. Sialic acid (e.g., Neu5Ac) is an important member of TACAs. Its overexpression on the surface of various cancer cells is closely associated with tumor invasion, metastasis, immune evasion, and poor prognosis.
The high-specificity, high-affinity Anti-Sialic Acid antibodies provided by AntibodySystem are key tools in cancer glycobiology research and the development of related targeted therapies. They can be used not only in basic research, such as detecting SA expression differences in different cells or tissues (as in this study), but also in immunohistochemical diagnostics, flow cytometric cell sorting, and efficacy evaluation of SA-targeted drugs. They hold significant importance for advancing the development of precise diagnostic and therapeutic strategies targeting the "tumor glyco-code."
|
Catalog |
Product Name |
|
RGK07801 |
Anti-Ganglioside GD2 Antibody (SAA0322) |
|
FHK33912 |
Anti-sLea/CA19-9 Antibody (1116NS19.9), PE |
|
FHK33910 |
Anti-sLea/CA19-9 Antibody (1116NS19.9) |
|
RGK27501 |
Anti-SSEA-3/GalGb4 Antibody (2A9) |
|
RGK28901 |
Anti-Ganglioside GM2 Antibody (E1) |
|
RGK11302 |
Anti-Ganglioside GD3 Antibody (R24) |
|
RGK11301 |
Anti-Ganglioside GD3 Antibody (SAA0321) |
|
RHK10008 |
Anti-Sialyl-Tn/STn Antibody (TKH2) |
|
DGK07805 |
Research Grade Anti-General Ganglioside GD2 Antibody (MORAb-028) |
