Antibodies are immune system-related proteins called immunoglobulins. Each antibody consists of four polypeptide – two heavy chains and two light chains joined to form a "Y" shaped molecule.
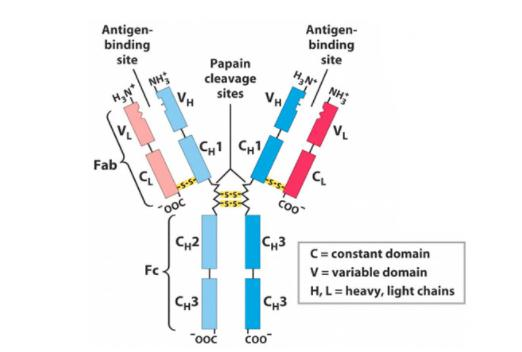
The amino acid sequence in the tips of the "Y" varies greatly among different antibodies. This variable region, composed of 110-130 amino acids, give the antibody its specificity for binding antigen. The variable region includes the ends of the light and heavy chains. Treating the antibody with a protease can cleave this region, producing Fab or fragment antigen binding that include the variable ends of an antibody.
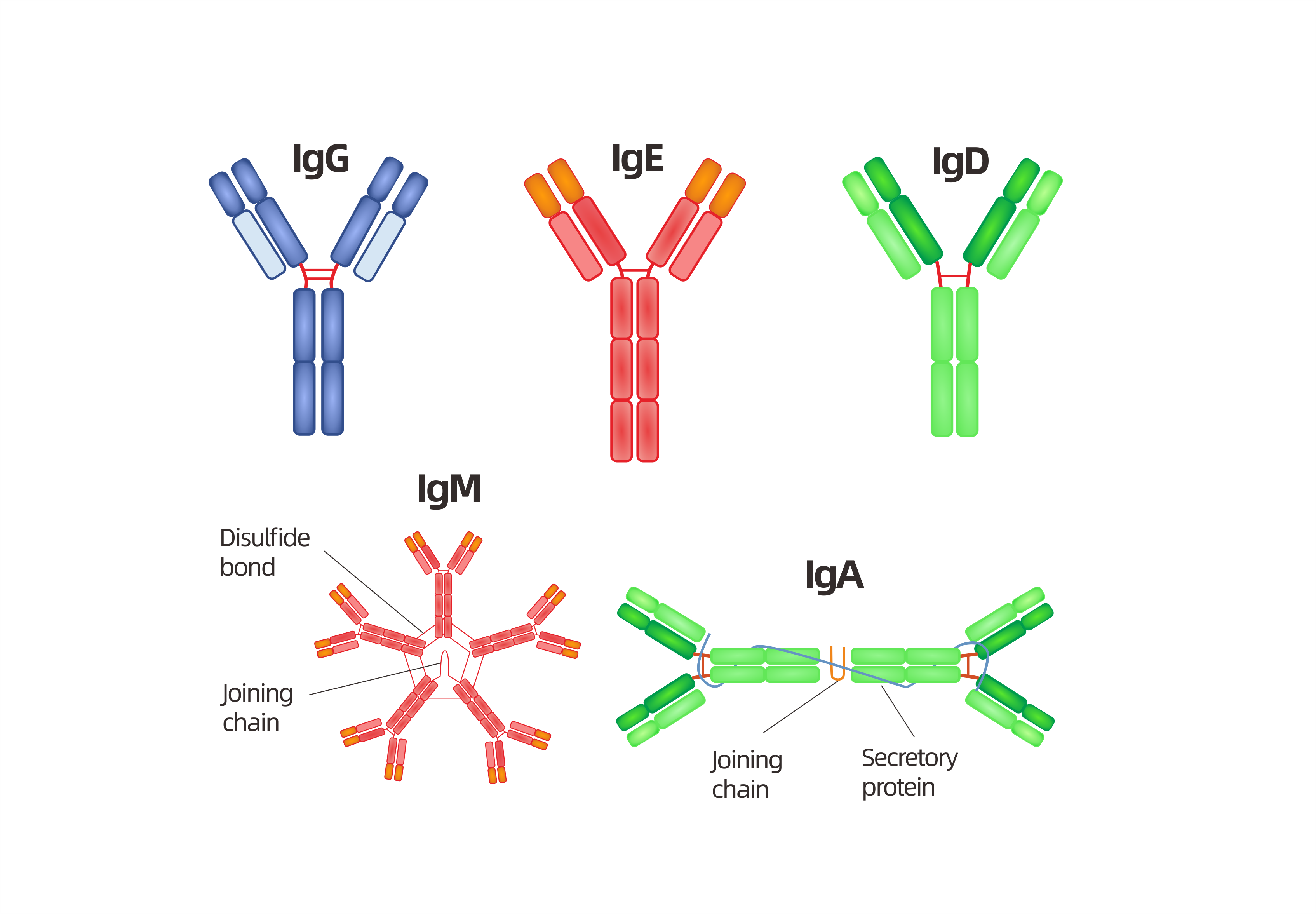
The constant region determines the mechanism used to destroy antigen. Antibodies are divided into five major classes, IgM, IgG, Iga, IgD, and IgE, based on their constant region structure and immune function.
L- chain:
1. L- chain of antibody is composed of about 220 aminoacids.
2. Around 100-110 aminoacids are located at N-terminal (amino-terminal) and the aminoacids sequences varies among antibodies. This region of L-chain is known as variable (V) region.
3. Remaining 110 aminoacids located at C-terminal (carboxyl-terminal) of L-chain are almost constant among antibodies. This region of L-chain is known as constant (C) region. Two types of constant region sequences are found ie. Lambda (λ) and Kappa (κ). In a particular antibody either2lambda or 2 kappa chains are present but not 1 lambda and kappa.
4. In human 60% light chain are kappa and 40% are lambda whereas in mice 95% of light chain are kappa and 5% are lambda.
H-chain:
1. In H-chain about 110 aminoacids are located at N-terminal which shows great variation among antibody. This region is known as Variable (V) region.
2. Remaining aminoacid sequences of H-chain is somewhat constant but reveals five different types of constant (C) heavy chain region ie. µ, α, δ, ε and γ.
3. The length of constant region of H-chain is 330 aminoacids for α, γ and δ and 440 aminoacids for µ and ε. Fab region 1. Antigen binding is accomplished by amino-terminal (N-terminal) region and effector functions by carboxyl terminal (C-terminal) region of antibody. 2. In an antibody molecule two Fab regions are found and they binds antigens. 3. Hypervariable region on L-chain (VL domain) and H-chain (VH domain) form antigen binding site.
4. Detailed comparison of aminoacids sequences of large number of VL and VH domain reveals that the sequence variation is concentrated in few discrete region of these domains. Thevariability plot of VH and VL domains shows maximum variation in certain region which is known as hypervariable region and this forms antigen binding site.
5. Antigen binding site is complementary to epitope of antigen, so it is also known as complementary determining regions (CDRs).
Fc region:
1. Fc region of immunoglobulin allows for interaction of immune complex with other phagocytic cells and complement.
2. Take parts in various biological functions that are determined by aminoacid sequences of each domains of constant region.
3. Many different form of Fc receptors exists.
Hinge region:
1. The γ, δ and α heavy chain contains an extended peptide sequence between CH1 and CH2 domain that has no homology with other domain, this region is known as hinge region.
2. Hinge region is rich in proline residue and is flexible. Therefore IgG, IgD and IgA are flexible.
3. The flexibility given by hinge region enable Fab region to assume various angle to bind antigen.
