In November 2025, multiple AntibodySystem products once again received broad recognition from the global research community. These products were cited in leading peer‑reviewed journals, including Nature Communications and Journal of Nanobiotechnology. The cited studies span a wide range of cutting‑edge research areas, such as the replication mechanisms of monkeypox virus, novel therapeutic strategies for psoriasis, FcγRI antibody–mediated cellular signaling, the synergistic effects of immune checkpoint blockade and ferroptosis, and the molecular basis of CREB1–DNA binding activity. Collectively, these publications highlight the critical value of AntibodySystem reagents in both fundamental life‑science research and translational medicine.
Looking ahead, AntibodySystem will continue to collaborate with research teams worldwide to support the entire research pipeline—from molecular mechanism elucidation to the identification of therapeutic targets—thereby advancing the frontiers of biomedical science. Today, together with Dr. Connie, we present selected publications from October/November 2025 that newly cited AntibodySystem products.

|
Title |
Preclinical assessment of two FcγRI-specific antibodies that competitively inhibit immune complex-FcγRI binding to suppress autoimmune responses |
|
Journal Information |
Nat Commun. 2025 Nov 19;16(1):10068. |
|
Cited products |
|
|
Catalog |
Product Name |
|
FHC93410 |
Anti-Human CD64/FCGR1A Antibody (H22) |
Key finding:
Hyperactivation of FcγRI is implicated in autoimmune disease, yet specific inhibitors have been limited. This study identified two antibodies, C01 and C04, that bind the IgG-binding domain of FcγRI with high affinity and displace ~60% of pre-bound immune complexes without receptor activation. In autoimmune models, they inhibited immune complex binding to monocytes and reduced IgG-mediated platelet consumption in vivo, with Fab-mediated blockade supporting their therapeutic potential.
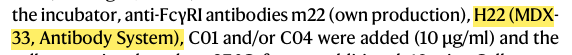
The study employed AntibodySystem’s Anti‑Human FcγRI (CD64) Antibody (H22)( Catalog: FHC93410) as a positive control in in‑vitro calcium flux assays. The results showed that H22 efficiently cross‑linked and activated FcγRI, triggering robust intracellular calcium mobilization, whereas the newly developed antibodies C01 and C04 did not induce receptor activation under identical conditions.

|
Title |
Self-assembled phenylboronic acid nanomedicine targets sialic acid to synergistically activate ferroptosis via RRM1 suppression and GPX4 Inhibition for precision colon cancer therapy |
|
Journal Information |
J Nanobiotechnology. 2025 Nov 25;23:735. |
|
Cited products |
|
|
Catalog |
Product Name |
|
RGK29702 |
Anti-Neu5Ac/O-sialic acid/Polysialic acid Antibody (Ab735) |
Key finding:
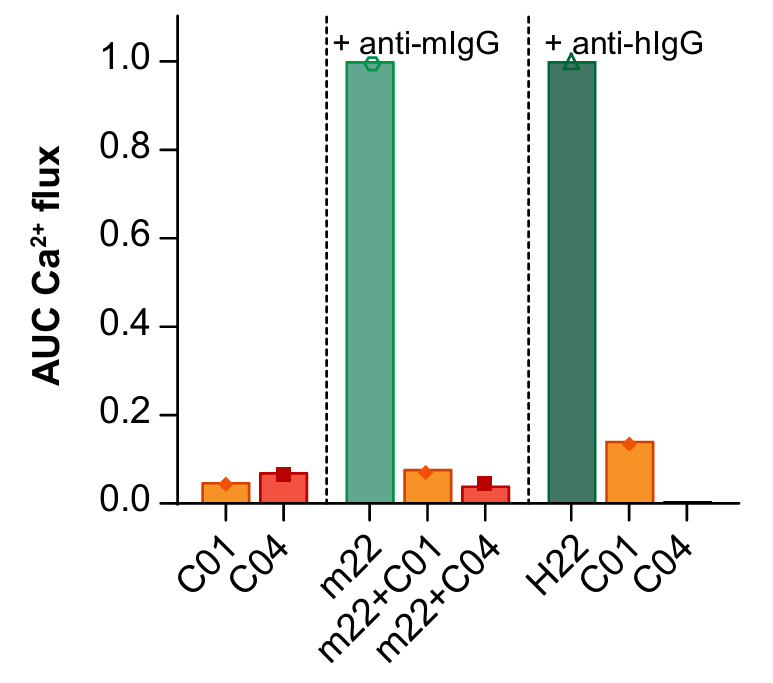
Hyperactivation of FcγRI contributes to multiple autoimmune diseases, but specific blocking antibodies have been lacking. This study developed two antibodies, C01 and C04, that bind with high affinity to the IgG-binding domain of FcγRI and displace approximately 60% of pre-bound immune complexes without triggering receptor activation. In autoimmune models, these antibodies inhibited immune complex binding to monocytes and reduced IgG-mediated platelet consumption in vivo. Structural analyses showed that FcγRI blockade is mediated by the Fab region, highlighting the therapeutic potential of C01 and C04.

AntibodySystem’s Anti‑Neu5Ac/O‑sialic acid/Polysialic Acid Antibody (Catalog: RGK29702) was used in Western blot assays as a core detection reagent for in‑vitro sialic‑acid expression analysis. The results demonstrated significantly higher levels of sialic acid on the surface of colon cancer cells compared with normal cells, providing a critical experimental basis for the design of subsequent phenylboronic‑acid‑based targeted nanomedicines.
|
Title |
A single non-coding SNP in FPGS modulates folate drug efficacy in acute lymphoblastic leukemia: data-driven exploration and experimental validation |
|
Journal Information |
Mol Biomed. 2025 Nov 21;6:114. |
|
Cited products |
|
|
Catalog |
Product Name |
|
YHD15901 |
Recombinant Human CREB1 Protein, N-His |
Key finding:
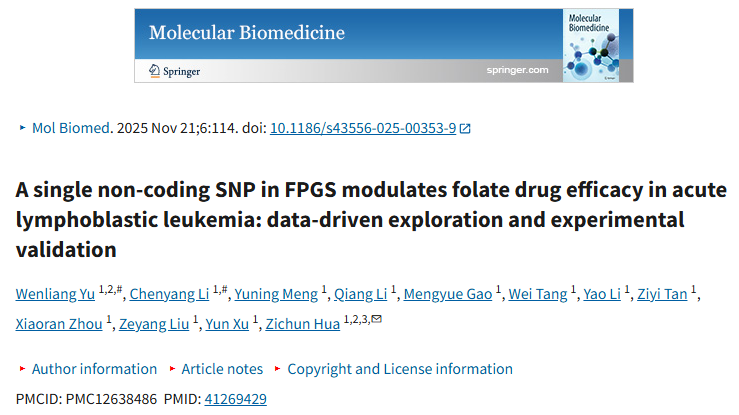
Methotrexate (MTX) remains a first-line maintenance therapy for acute lymphoblastic leukemia (ALL), but its efficacy is influenced by genetic variation. This study identified a non-coding SNP in the FPGS gene (rs1544105) that is strongly associated with MTX response, with the AA genotype linked to poorer outcomes compared with GG. Prime-editing experiments demonstrated that the rs1544105 A>G substitution increased FPGS expression, enhanced intracellular MTX retention, and improved MTX efficacy in cellular and animal models. Mechanistically, this variant strengthened CREB1 binding to the FPGS regulatory region, providing a molecular basis for personalized MTX therapy in ALL.

The article references the Recombinant Human CREB1 Protein, N-His (Catalog: YHD15901) from AntibodySystem, which was used in electrophoretic mobility shift assay (EMSA) experiments to validate the binding ability of the transcription factor CREB1 to a DNA sequence containing the rs1544105 locus. The results demonstrated that the recombinant CREB1 protein exhibited stronger binding to probes containing the G allele of rs1544105. This suggests that the mutation enhances the affinity of CREB1 for the FPGS promoter, thereby upregulating FPGS transcription.

|
Title |
Characterization of Human Sertoli Cells Infected With Monkeypox Virus |
|
Journal Information |
J Med Virol. 2025 Nov;97(11):e70661. |
|
Cited products |
|
|
Catalog |
Product Name |
|
PVV14801 |
Anti-Monkeypox virus/MPXV F3L Polyclonal Antibody |
|
RVV13201 |
Mouse Anti-Monkeypox virus/MPXV E8L Antibody (SAA0285) |
Key finding:
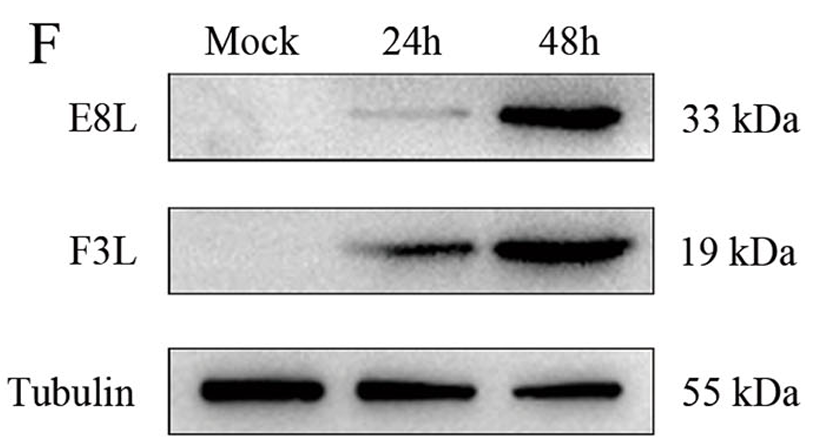
Although the 2022 monkeypox outbreak showed features of sexual transmission, MPXV infection in testicular cells remains poorly understood. This study demonstrates that MPXV infects human Sertoli cells, inducing cellular lesions and active viral replication through cytoplasmic viral factories and exocytotic release. Transcriptomic analyses revealed upregulation of stress, inflammatory, and cell-cycle pathways, along with suppression of antiviral genes, defining a host-response landscape that may underlie MPXV-associated epididymo-orchitis.

This study employed AntibodySystem’s Anti‑MPXV F3L Polyclonal Antibody (Catalog: PVV14801) and Mouse Anti‑MPXV E8L Antibody (Catalog: RVV13201) in Western blot analyses to assess viral protein expression in human Sertoli cells following MPXV infection. The results revealed time‑dependent increases in the expression of viral F3L and E8L proteins, confirming their reliability and specificity for detecting MPXV infection in human Sertoli cells.

|
Title |
Ergosterol, a Novel RORγt Inverse Agonist, Enhances Skin Barrier Function and Reduces Skin Inflammation in IMQ-Induced Mice With Psoriasis |
|
Journal Information |
Arch Pharm (Weinheim). 2025 Nov;358(11):e70121. |
|
Cited products |
|
|
Catalog |
Product Name |
|
YHE81603 |
Recombinant Human RORC/NR1F3/RORgT Protein, N-His |
Key finding:
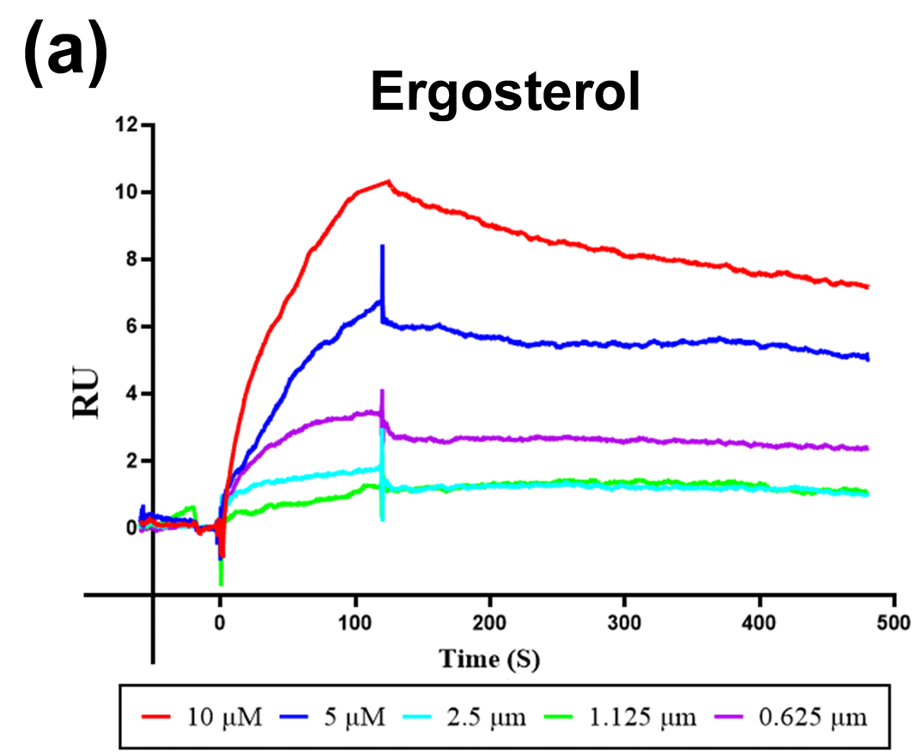
RORγt is a key nuclear receptor transcription factor driving IL-17⁺ T helper cell differentiation and is an emerging therapeutic target in inflammatory diseases such as psoriasis. This study identified ergosterol (ERG) as a novel RORγt inverse agonist that suppresses RORγt activity, reduces pro-inflammatory cytokine production, and improves skin pathology in IMQ-induced psoriasis models. ERG also restored epidermal barrier function by upregulating tight junction proteins, highlighting its potential as a therapeutic agent for psoriasis.

AntibodySystem’s Recombinant Human RORC/NR1F3/RORγt Protein, N‑His (Catalog: YHE81603) was used in surface plasmon resonance assays (SPR) to characterize the interaction between ergosterol and the RORγt transcription factor. The results demonstrated that ergosterol specifically binds to RORγt with an equilibrium dissociation constant (Kd) of 6.12 ± 1.35 μM, confirming a direct molecular interaction. This binding underpins the compound’s subsequent anti‑inflammatory effects and its ability to restore skin barrier function in psoriasis models.