The theme for World Diabetes Day on November 14, 2025, is "Diabetes and Well-being," shifting the focus beyond glycemic management to encompass psychological health, social adaptation, and quality of life enhancement. This theme also charts the course for cutting-edge research, emphasizing that through deciphering disease mechanisms and optimizing diagnostic and therapeutic strategies, we can ultimately elevate the overall well-being of people living with diabetes.
Diabetes mellitus is a metabolic disease that is marked by persistent hyperglycemia due to inadequate insulin secretion or insulin resistance. Its prevalence is increasing yearly. Diabetes mellitus can lead to serious health complications that are the primary cause of mortality and disability among diabetic patients, including diabetic retinopathy, diabetic foot ulcers, diabetic peripheral neuropathy, and diabetic periodontitis, and so on. Traditional treatments for diabetes and its complications still suffer from limited clinical efficacy and high therapeutic side effects. Diabetes has become a worldwide health burden due to its high incidence, disability and mortality, which is estimated to be the eighth leading cause of death combined disability.
Diabetes is a heterogeneous syndrome characterized by defined hyperglycemia which is classified as type 1 diabetes (T1D), type 2 diabetes (T2D), specific types of diabetes and gestational diabetes mellitus. T2D accounts for 96% of diabetes and is one of the important noncommunicable chronic diseases that seriously threaten human health.
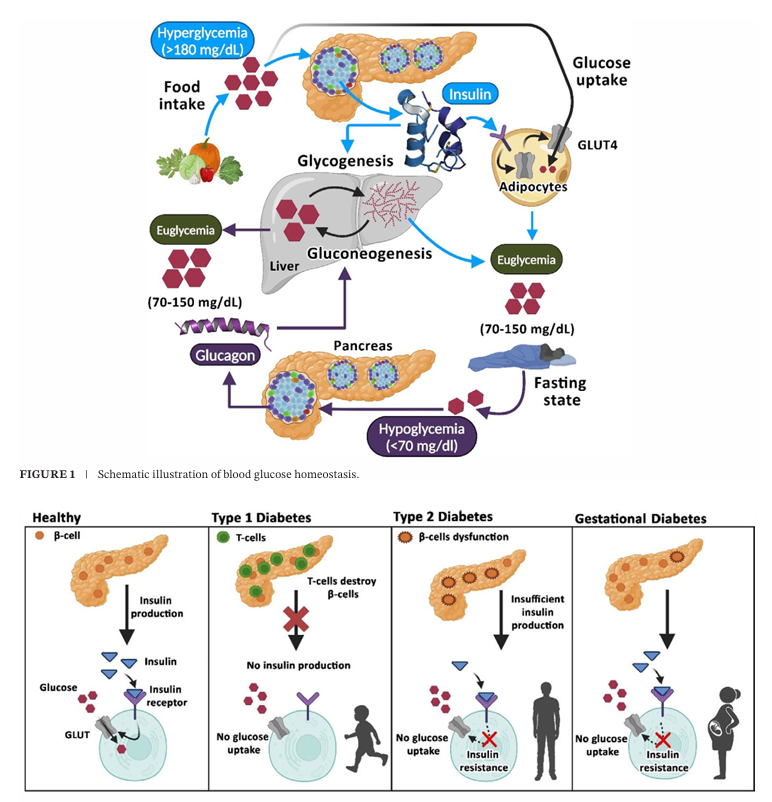
Fig 1. Schematic representation of different types of diabetes mellitus(Compr Physiol. 2025 Feb;15(1):e70003.)
Type 1 diabetes,T1D
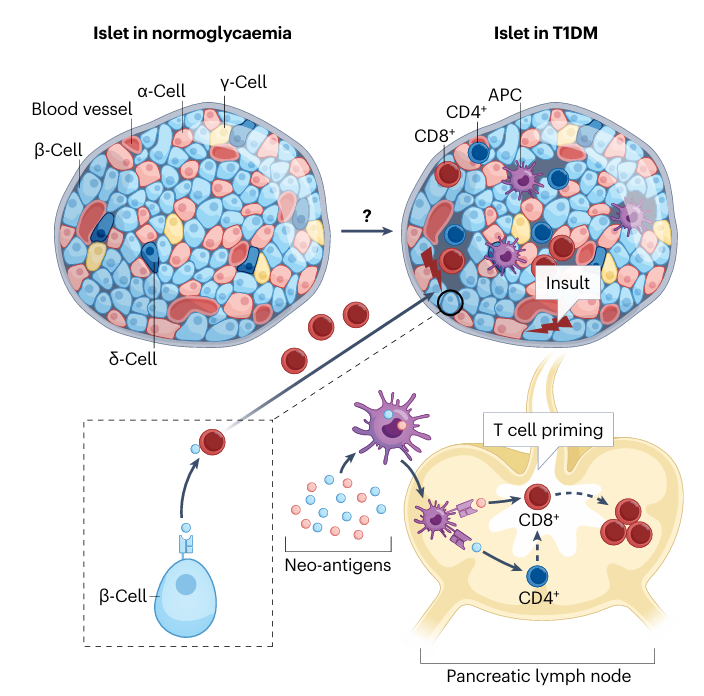
Fig 2. Major postulated mechanisms of pancreatic β-cell damage and destruction(Nat Rev Endocrinol. 2025 Oct;21(10):608-622.)
T1D is an autoimmune disease in which aberrant activation of the immune system leads to targeted destruction of pancreatic β‑cells. Patients therefore depend on lifelong exogenous insulin to survive; without it they rapidly develop severe hyperglycaemia and life‑threatening diabetic ketoacidosis. Globally, the incidence of T1D is rising year after year, especially among children and adolescents, who account for 80 %–90 % of all diabetes cases in this age group and are therefore often referred to as “juvenile diabetes.” Epidemiological projections suggest that by 2040 the total number of individuals with T1D will increase by at least 66 %, imposing a heavy economic and health burden on families and societies worldwide.
Therapeutic strategies for T1D have shifted from pure insulin replacement toward a multimodal approach that combines immune modulation with β‑cell regeneration. In the immune‑intervention arena, several agents are currently in clinical evaluation: the anti‑CD3 monoclonal antibody Teplizumab (the only FDA‑approved drug for delaying disease onset in high‑risk individuals, with an average postponement of about three years), the CTLA‑4‑Ig co‑stimulation blocker abatacept, the anti‑CD20 monoclonal antibody rituximab, IL‑6‑receptor or TNF‑α antagonists, and the JAK inhibitor baricitinib, among other biologics and small‑molecule agents. Non‑specific immunosuppressants such as cyclosporine and azathioprine, as well as antigen‑specific immunotherapies (e.g., oral or intranasal insulin, glutamic acid decarboxylase preparations), are also being explored in high‑risk cohorts. Current research trends favour combination regimens—e.g., Teplizumab together with low‑dose IL‑2—to enhance tolerability and prolong therapeutic durability.
On the cell‑replacement front, pancreas or islet transplantation, β‑cell grafts derived ex‑vivo, and mesenchymal stem‑cell transplantation are being investigated in specialised centres with the aim of restoring endogenous insulin secretion. In parallel, induced pluripotent stem‑cell‑derived β‑like cells (e.g., VX‑880/VX‑800) have entered Phase I/II trials, and a subset of participants have achieved insulin independence accompanied by measurable C‑peptide recovery. Comprehensive disease management still relies on intensified insulin therapy—multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) integrated with closed‑loop (“artificial pancreas”) systems—augmented by continuous glucose monitoring (CGM) for real‑time glycaemic adjustment. Individualised glycaemic targets, structured diabetes education, and tailored nutrition and exercise programmes together constitute a holistic care model aimed at minimising both microvascular and macrovascular complications. Overall, future breakthroughs are expected to arise from the synergistic integration of immune modulation, β‑cell regeneration, and precision patient stratification, moving the field from mere symptom control toward true disease modification.
Type 2 diabetes,T1D
T2D is a heterogeneous disease characterized by progressive loss of pancreatic β-cell insulin secretion, typically developing after the onset of insulin resistance (IR). As an integral component of metabolic syndrome (MS), it has been termed Metabolic Dysfunction Syndrome (MDS). The pathogenesis of T2D remains incompletely understood, with both insulin resistance and β-cell dysfunction playing central roles in the pathophysiology.
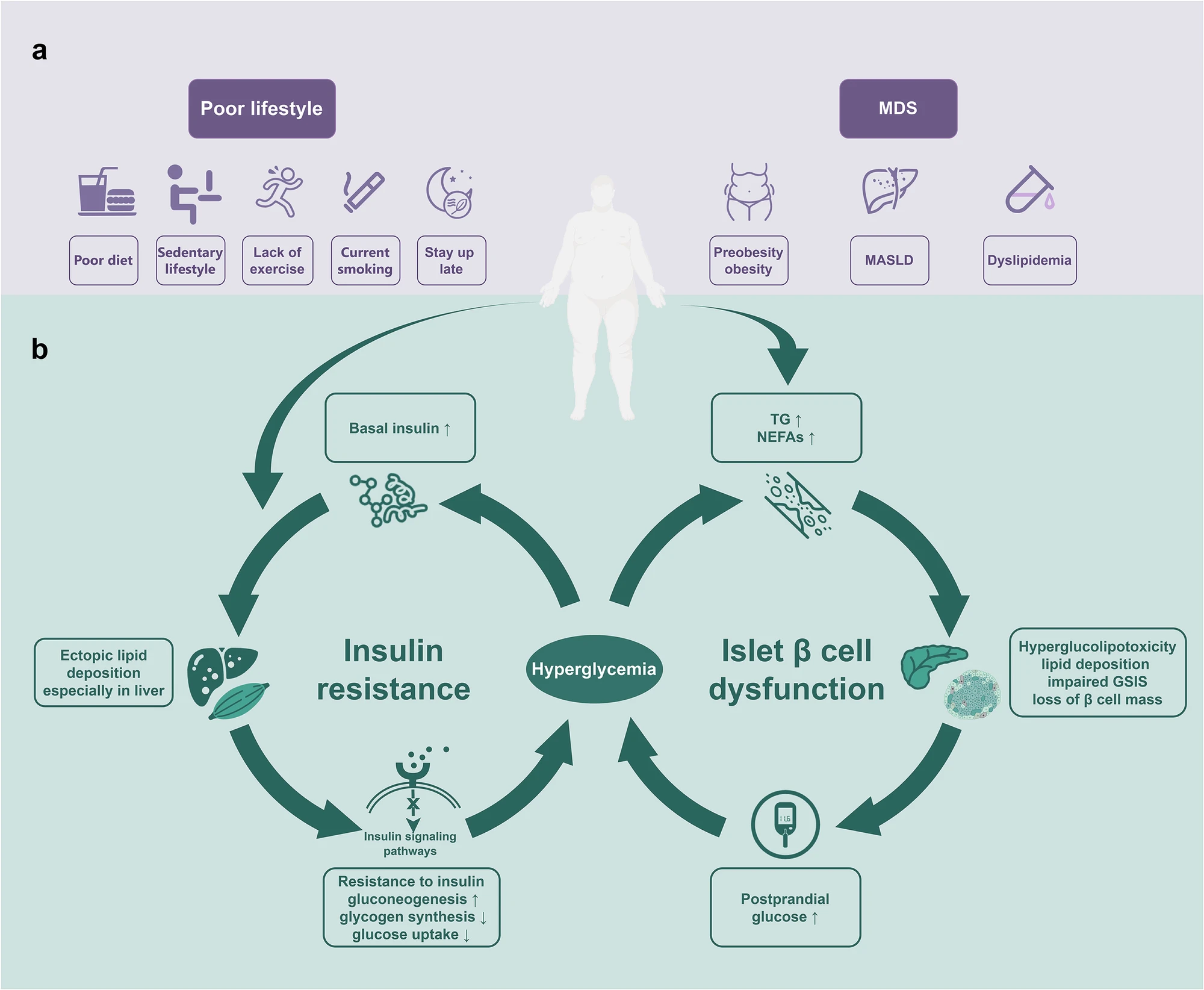
Fig 3. T2D in adults: pathogenesis, prevention and therapy(Signal Transduct Target Ther. 2024 Oct 2;9(1):262.)
The continuous hyperglycemia can induce target organ damage (TOD) by increasing the risk of panvascular disease, including microvascular disease (such as diabetic retinopathy, nephropathy and neuropathy), and atherosclerotic macrovascular disease (cardiovascular, cerebrovascular, and other peripheral vascular diseases). Unlike classical T1D with almost only hyperglycemia, T2D is just one component of MDS, and is often accompanied by other components of MDS, such as overweight/obesity (preobesity might be a more appropriate term instead of “overweight” since obesity is not determined only by weight), metabolic dysfunction associated steatotic liver disease (MASLD), dyslipidemia. They are usually considered as the upstream diseases of T2D. Among them, preobesity/obesity, MASLD and some coexisting hypertension, closely related to poor lifestyle, are independent risk factors for the development of cardiovascular-kidney-metabolic syndrome and T2D,2 indicating their preventable characteristic.
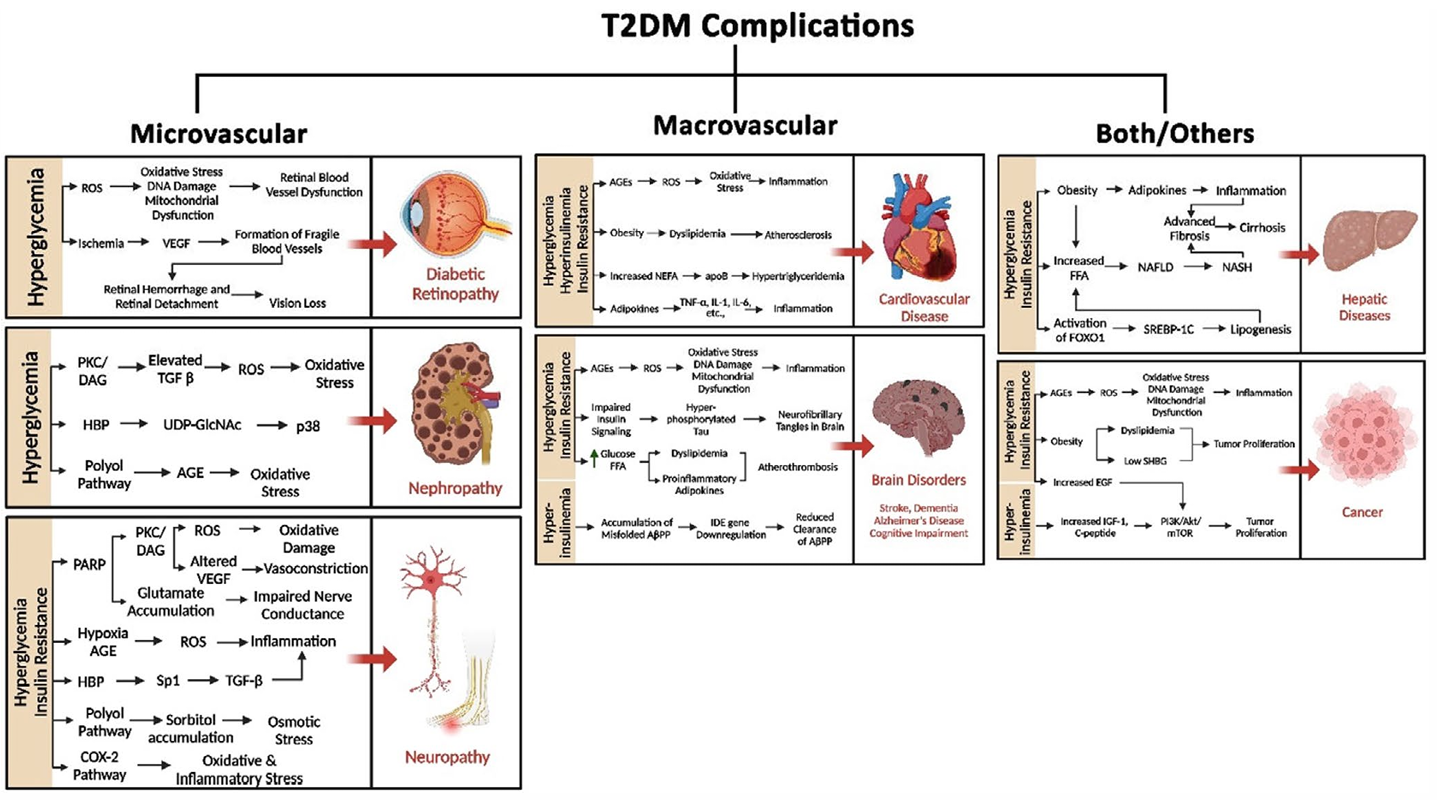
Fig 4. Common health issues that are often found to be associated with obese patients diagnosed with chronic T2D (Compr Physiol. 2025 Feb;15(1):e70003.)
Current pharmacological management of type 2 diabetes (T2D) has evolved from a singular focus on glycemic control to a comprehensive, individualized strategy built upon three fundamental pillars: glycemic efficacy, weight management, and cardiorenal risk control.
For patients without established cardiovascular disease (CVD), chronic kidney disease (CKD), or other high-risk factors, metformin and other agents with proven glucose-lowering efficacy remain foundational options. When more potent glycemic reduction is required, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) such as dulaglutide and semaglutide, the dual GIP/GLP-1 receptor agonist tirzepatide, insulin, or combination regimens can be considered. For those with overweight or obesity, semaglutide and tirzepatide have demonstrated significant benefits in promoting weight loss.
In patients with established atherosclerotic cardiovascular disease (ASCVD), heart failure (HF), CKD, or those at high risk for these conditions, drug selection should prioritize agents with proven cardiorenal benefits. Specifically, GLP-1 RAs and sodium-glucose cotransporter-2 inhibitors (SGLT2is) are recommended as first-line choices for individuals with ASCVD or at high ASCVD risk. For patients with HF, SGLT2is are the preferred class, while in CKD, SGLT2is are also recommended, with GLP-1 RAs serving as an alternative if SGLT2is are not suitable. When glycemic targets are not achieved with monotherapy, combination therapy utilizing agents with complementary mechanisms of action is essential for intensifying treatment.
These advances in therapeutic philosophy are driven by the emergence of innovative drug classes. Novel agents, including GLP-1 RAs, SGLT2is, and dual GIP/GLP-1 receptor agonists, not only provide superior glycemic control but also offer additional metabolic benefits such as weight reduction and cardiorenal protection. This progression signifies a paradigm shift in T2D management—from a conventional "glucose-centric" approach to a new era characterized by "multi-target intervention and systematic management."
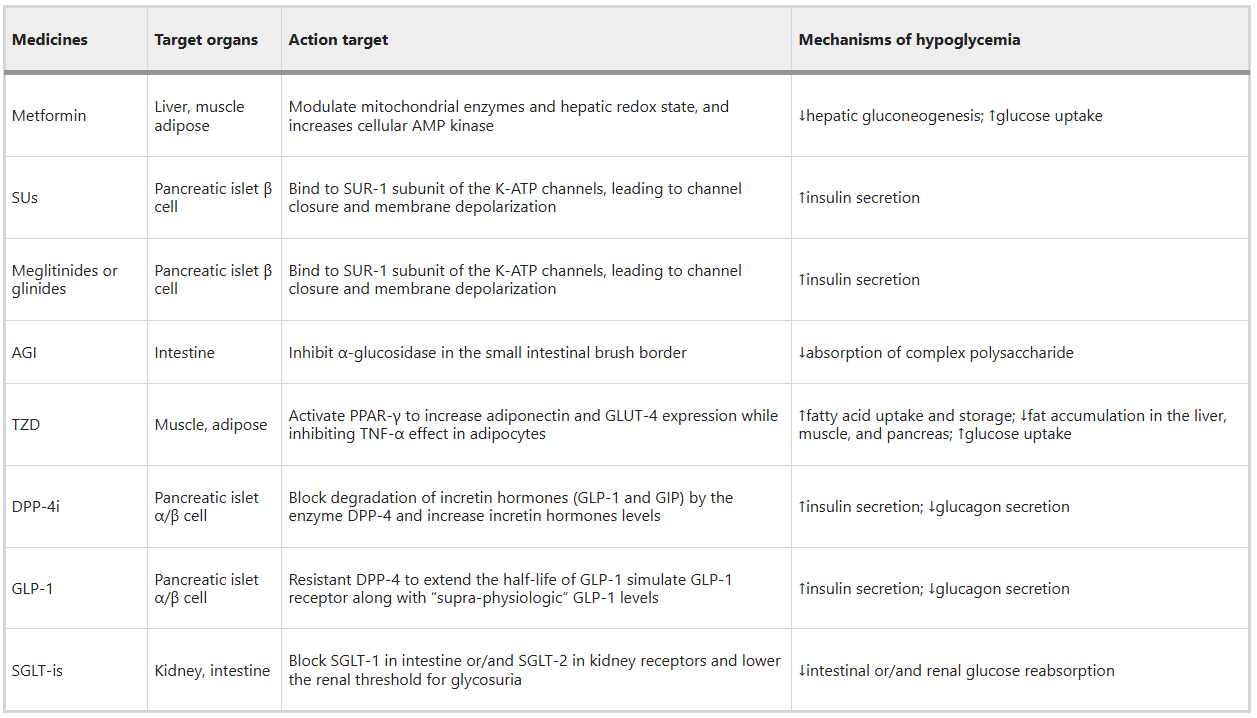
Fig 5. Targets and mechanisms of hypoglycemic agents (Signal Transduct Target Ther. 2024 Oct 2;9(1):262.)
References
1. Lu X, Xie Q, Pan X, et al. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduction and Targeted Therapy. 2024;9(1):262.
2. Singh A, Shadangi S, Gupta PKr, Rana S. Type 2 Diabetes Mellitus: A Comprehensive Review of Pathophysiology, Comorbidities, and Emerging Therapies. Comprehensive Physiology. 2025;15(1).
3. Ansari MA, Chauhan W, Shoaib S, et al. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. International Journal of Obesity. Published online September 11, 2023:1-21.
4. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 Diabetes Mellitus. Nature Reviews Disease Primers. 2015;1(1).
AntibodySystem provides diabetes-related products, delivering more tools and solutions for diabetes research.
Antibody
|
Catalog |
Product Name |
|
RHK13902 |
Anti-Semaglutide (GLP-1 analogue) Antibody (SAb2275) |
|
RHK13901 |
Anti-Semaglutide (GLP-1 analogue) Antibody (SAb2274) |
|
PHK13901 |
Anti-Semaglutide (GLP-1 analogue) Polyclonal Antibody |
|
PHB93502 |
Anti-Dulaglutide Polyclonal Antibody |
|
RHH01904 |
Anti-RAGE/AGER Antibody (R3T53) |
|
PHH01901 |
Anti-AGER Polyclonal Antibody |
|
PHK13902 |
Anti-Tirzepatide Polyclonal Antibody |
|
DHC27703 |
Research Grade Teplizumab |
|
DHE03429 |
Research Grade Abatacept |
|
DHE40129 |
Research Grade Dulaglutide |
|
DPE40105 |
Research Grade Semaglutide |
ELISA Kit
|
Catalog |
Product Name |
|
KAC90701 |
Anti-Rituximab ELISA Kit |
|
KDK13903 |
Tirzepatide (LY3298176) ELISA Kit-HS |
|
KDE40103 |
Dulaglutide ELISA Kit |
|
KAK13901 |
Anti-Semaglutide ELISA Kit |
|
KAK13903 |
Anti-Semaglutide Neutralizing Antibody ELISA Kit |
|
KDK13901 |
Semaglutide ELISA Kit |
