Globally, measles cases are trending upwards in North America, the European Union, and the United Kingdom. The United States is currently facing its most severe measles outbreak since declaring the disease eliminated in 2000. According to the latest data released by the Centers for Disease Control and Prevention (CDC) on the 22nd, the U.S. has reported 1,618 confirmed measles cases so far this year, resulting in 198 hospitalizations and 3 deaths. This marks the highest number of confirmed cases in the past 33 years, surpassing the previous record of 1,274 cases set in 2019. The outbreak primarily affects children under five years of age, with approximately 92% of patients being unvaccinated or having an unknown vaccination status.

Figure 1. Centers for Disease Control and Prevention (CDC)
Data from the Canadian health authorities indicate that as of September 20th, Canada has reported over 5,000 measles cases this year, including two infant deaths. The provinces of Alberta and Ontario are the most severely affected, with the majority of infections occurring among children and infants. Although Canada's population is significantly smaller than that of the U.S., its recent case count is nearly three times higher. Canadian health officials have identified lack of vaccination as the primary driver of this large-scale outbreak.

Figure 2. Health Canada
Measles is a highly contagious acute febrile rash illness caused by the measles virus (MeV). MeV belongs to the genus Morbillivirus within the family Paramyxoviridae. It is an enveloped virus with a non-segmented, single-stranded, negative-sense RNA genome approximately 16 kb in length. The measles virus encodes six structural genes, which produce six structural proteins: the nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin (H), and large protein (L), as well as two non-structural proteins, V and C.
The hemagglutinin (H) glycoprotein, one of two transmembrane glycoproteins on the viral surface, mediates attachment to host cell receptors. These receptors include Signaling Lymphocyte Activation Molecule (SLAM or CD150), found on lymphocytes, monocytes, macrophages, and dendritic cells, and NECTIN-4, an adherens junction component of epithelial cells. The distribution of these receptors determines the cell types and tissues susceptible to measles virus infection. Lifelong immunity following measles infection is primarily attributed to neutralizing IgG antibodies targeting the H protein, which block the virus from binding to host cell receptors. The other viral surface glycoprotein, the fusion (F) protein, mediates fusion of the viral envelope with the host cell membrane, allowing the viral ribonucleoprotein complex to enter the cytoplasm.
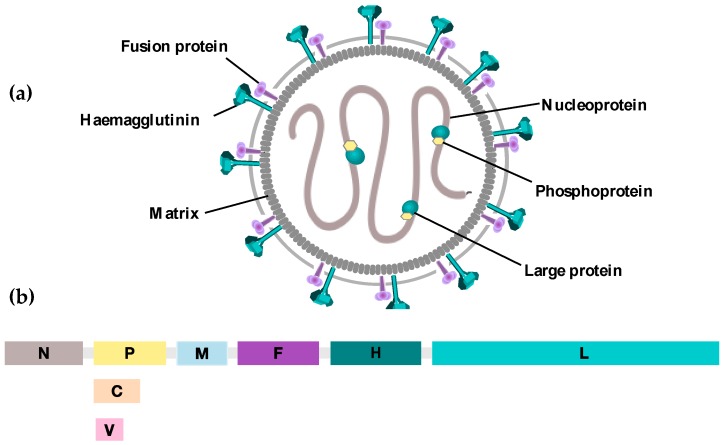
Figure 3. Schematic representation of measles virus(Viruses. 2016 Oct 22;8(10):294.)
Measles is considered one of the most contagious viruses in the world, even exceeding the transmissibility of SARS-CoV-2. The virus spreads primarily through respiratory droplets from secretions, but can also be transmitted via direct contact with infected nasopharyngeal secretions. When an infected person coughs or sneezes, the virus, contained in droplets, enters susceptible individuals through the mouth, nose, throat, or conjunctiva. After inhalation, the virus initially infects immune cells in the respiratory tract, subsequently disseminates throughout the body, and ultimately replicates in respiratory epithelial cells by utilizing the NECTIN-4 receptor, a crucial step for viral shedding back into the airways. Infected individuals are highly contagious for several days before and after the rash appears. On average, a single person with measles can spread the virus to 9-18 others, leading to extremely efficient transmission and a high potential for large-scale outbreaks.
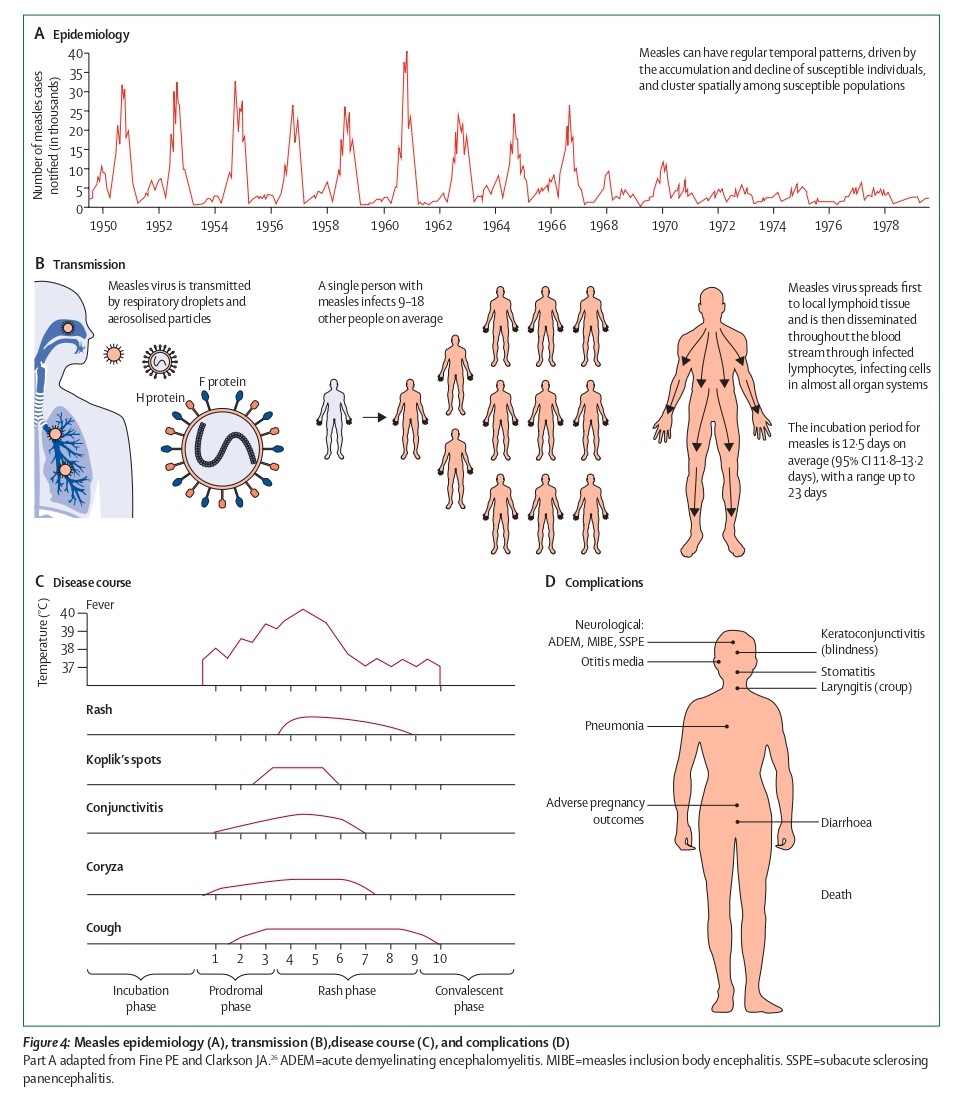
Figure 4. Measles epidemiology (A), transmission (B), disease course (C), and complications (D)(Nat Rev Dis Primers. 2016 Jul 14:2:16049.)
Typical symptoms of measles virus infection include fever, a maculopapular rash, and often cough, coryza (runny nose), and conjunctivitis. The measles virus induces immune suppression, and its severe sequelae can include pneumonia, gastroenteritis, blindness, measles inclusion body encephalitis, and subacute sclerosing panencephalitis (SSPE). Confirmation of measles relies on clinical presentation and laboratory testing, including detection of measles virus-specific IgM antibodies and/or viral RNA.
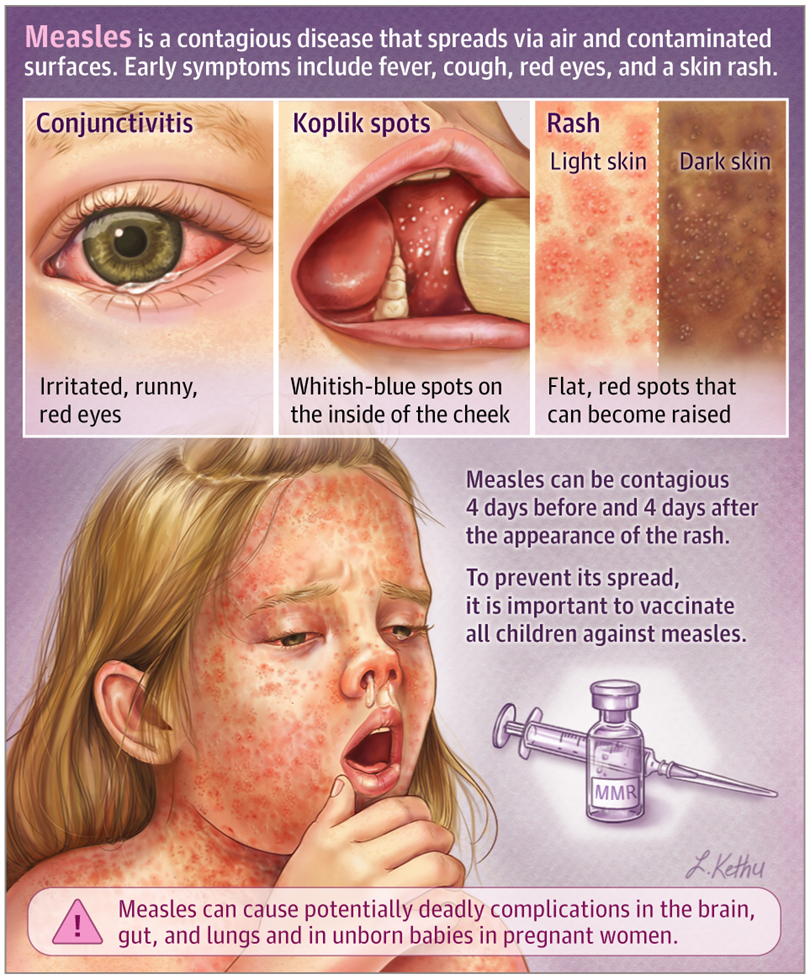
Figure 5. Measles symptoms(JAMA dermatology. 2019;155(12):1436.)
Currently, the only available vaccines against measles are live attenuated vaccines. These include measles-only vaccines, measles-rubella (MR) vaccine, measles-mumps (MM) vaccine, measles-mumps-rubella (MMR) vaccine, and measles-mumps-rubella-varicella (MMRV) vaccine. Collectively, these are referred to as measles-containing vaccines (MCVs). Despite the global availability of safe and highly effective measles vaccines, with two doses providing up to 97% protection, the reality remains concerning. Global coverage for the first dose of measles vaccine has stagnated at around 83%, well below the 95% threshold required for herd immunity. This immunity gap has directly contributed to severe resurgences of measles in many parts of the world. In 2024, Europe reported its highest number of cases in 25 years, while in the U.S., 99% of cases this year occurred in unvaccinated individuals or those with unknown vaccination status. The primary drivers are immunization disruptions caused by the COVID-19 pandemic, the growing phenomenon of "vaccine hesitancy," and religious beliefs. Suboptimal vaccine coverage has led to the recurrence and resurgence of measles, even re-establishing endemic transmission in countries where measles had previously been eliminated (e.g., Venezuela, Brazil, and Mongolia).
Achieving the global goal of measles elimination now depends not only on the vaccine itself but, more urgently, on strengthening primary healthcare systems and combating misinformation to increase vaccination coverage.

Figure 6. Measles vaccination(JAMA Pediatrics. 2018;172(9):896.)
Research on the measles virus is evolving from traditional live attenuated vaccines towards new technologies. Efforts include using human SLAM knock-in mouse models and ferret models to better understand viral pathogenesis; exploring antiviral drugs such as nucleoside polymerase inhibitors and fusion inhibitory peptides; and advancing innovative vaccine platforms like microarray patches, mRNA vaccines, and recombinant viral vectors to improve vaccination coverage and immune durability. Concurrently, wastewater genomic surveillance and whole-genome sequencing are being used to track viral trends. Future priorities will focus on high-throughput drug screening, evaluating long-term immunity, elucidating the mechanisms of neurological complications, and developing multifunctional vaccine vectors, aiming for breakthroughs in enhancing protection, shortening the treatment window, and ultimately eradicating measles.
References
1. Moreno MA. Measles. JAMA Pediatrics. 2018;172(9):896.
2. Balu B, Mostow EN. Measles. JAMA dermatology. 2019;155(12):1436.
3. Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nature Reviews Disease Primers. 2016;2(1).
Based on the virological and immunological research background outlined above, AntibodySystem offers a range of tools, including measles virus-related recombinant proteins and corresponding antibodies, to accelerate basic research and the development of prevention and treatment strategies, thereby supporting global measles virology research.
|
Catalog |
Product Name |
|
EVV33901 |
Recombinant MeV H/Hemagglutinin glycoprotein Protein, C-His |
|
YVV33901 |
Recombinant MeV H/Hemagglutinin glycoprotein Protein, N-His |
|
YVV34501 |
Recombinant MeV F1/Fusion glycoprotein F1 Protein, N-His |
|
YVV34601 |
Recombinant MeV N/Nucleoprotein Protein, N-His |
|
YVV34701 |
Recombinant MeV M/Matrix protein Protein, N-His |
|
YVV34801 |
Recombinant MeV P/Phosphoprotein Protein, N-His |
|
YVV34901 |
Recombinant MeV V/Non-structural protein V Protein, N-His |
|
YVV35001 |
Recombinant MeV C/Protein C Protein, N-His |
