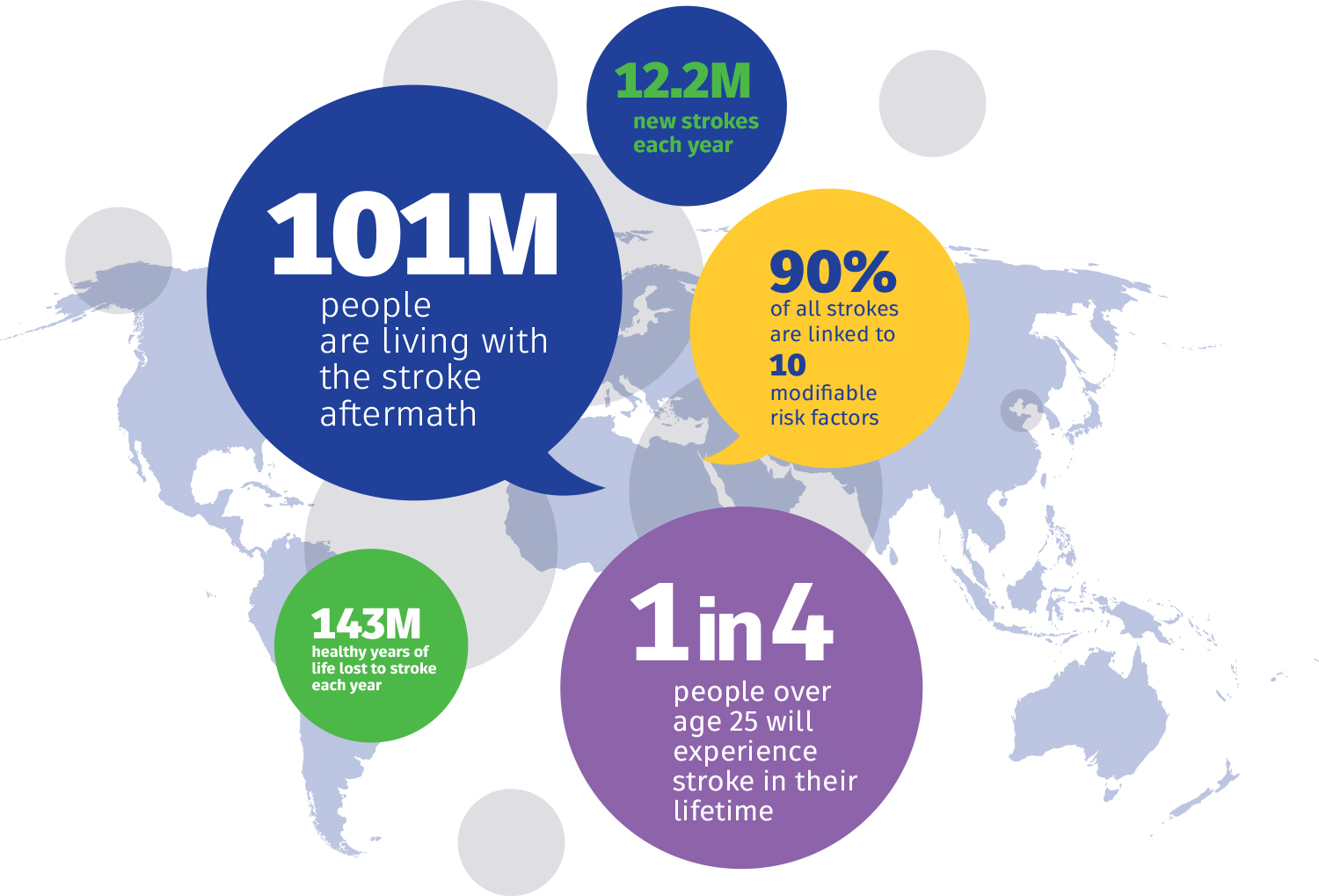
October 29th each year marks World Stroke Day. Established by the World Stroke Organization (WSO), it was initiated in 2006 and officially launched in 2007. This day aims to raise global awareness about stroke—a preventable and treatable disease—and to mobilize governments, healthcare institutions, and the public to take preventive and treatment measures. The global core slogan set by WSO for 2025 is "Every Minute Counts. Know the signs of stroke. Act FAST."
Stroke refers to an interruption of blood flow to the brain due to a sudden blockage or rupture of a cerebral blood vessel, clinically manifesting as sudden neurological deficits. According to WHO reports, stroke is the second leading cause of death globally and the third leading cause of death and disability. Its incidence, disability rate, and mortality rate remain high. Approximately 15 million people worldwide suffer a stroke each year, making it second only to heart disease.
Based on vascular pathology, stroke can be classified into two main types: Ischemic Stroke (Cerebral Ischemic Stroke, CIS, accounting for approximately 85%) and Hemorrhagic Stroke. The former is commonly known as "cerebral infarction," while the latter is known as "cerebral hemorrhage." Ischemic stroke occurs when a thrombus or arterial stenosis prevents blood from reaching the brain, causing brain tissue damage. Hemorrhagic stroke results from a ruptured blood vessel causing blood to leak into or around the brain tissue, compressing or damaging brain cells.
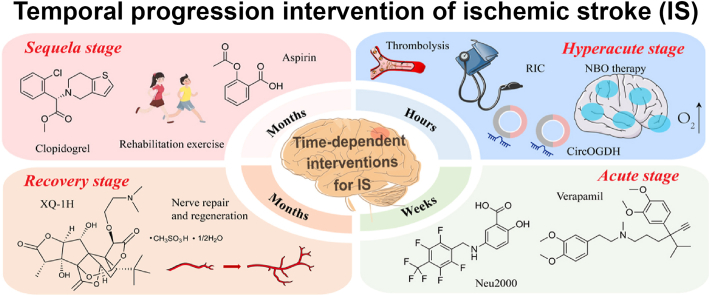
Figure 1. Temporal progression intervention of ischemic stroke(Acta Pharm Sin B. 2025 Jul 23;15(9):4543–4581.)
Acute Ischemic Stroke (AIS) accounts for approximately 70% of all strokes. It is a pathological event characterized by focal cerebral infarction due to arterial thrombosis, embolism, or severe hypoperfusion, leading to neurological deficits.
Signs and symptoms of stroke can include: sudden numbness or weakness in a limb, facial drooping, difficulty speaking or understanding speech, confusion, trouble with balance or coordination, and vision loss. Acute Ischemic Stroke is sometimes preceded by a Transient Ischemic Attack (TIA), a warning sign caused by a temporary disruption of blood flow to the brain, resulting in transient neurological deficits.
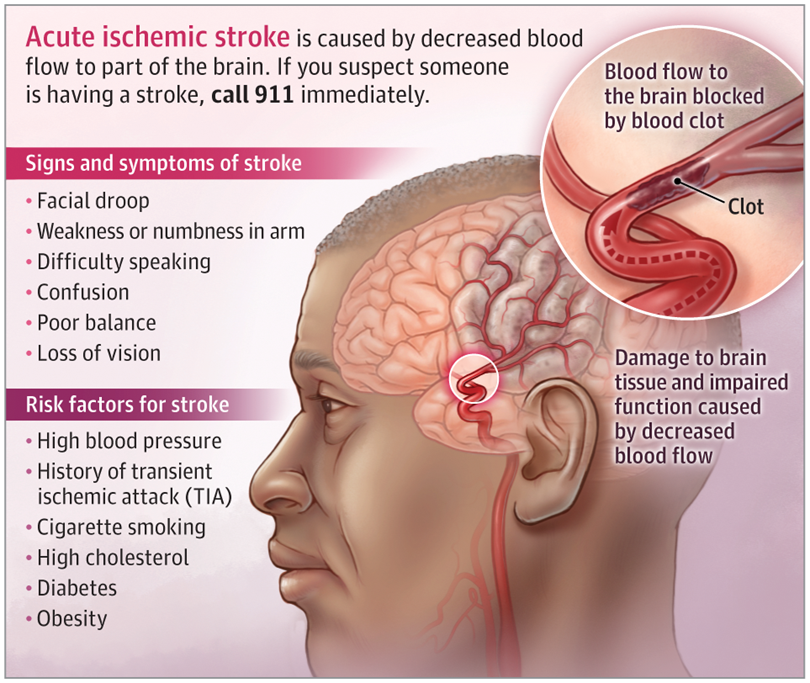
Figure 2. Acute Ischemic Stroke(JAMA. 2022 Mar 1;327(9):885.)
The primary risk factor for Acute Ischemic Stroke is hypertension. Other associated risk factors include a history of Transient Ischemic Attack (TIA), smoking, hypercholesterolemia, diabetes, obesity, end-stage renal disease, and cardiac arrhythmias such as atrial fibrillation. Acute Ischemic Stroke requires treatment as quickly as possible because earlier intervention leads to better outcomes. If diagnosed early (within 4.5 hours of symptom onset), some patients can receive intravenous thrombolytic therapy. The conventional tissue plasminogen activator (tPA) remains the first-line thrombolytic agent for acute ischemic stroke. This medication reduces the risk of long-term disability after an AIS but carries an approximately 6% risk of intracerebral hemorrhage. Furthermore, depending on the occluded artery, tPA can restore blood flow to the brain in approximately 10% to 50% of patients.
In stroke research, various biomarkers in blood and cerebrospinal fluid have been systematically utilized for early diagnosis, severity stratification, and prognosis assessment.
Markers of thrombosis and coagulation function primarily include Fibrinogen (FIB) and D-dimer. Fibrinogen is a key coagulation factor in plasma; elevated levels are closely associated with the risk of ischemic stroke and poor prognosis. D-dimer, a fibrin degradation product, reflects thrombotic activity in the body and is often elevated in patients with acute stroke, particularly hemorrhagic stroke. Regarding endothelial dysfunction and metabolic abnormalities, Asymmetric Dimethylarginine (ADMA) is considered an endogenous inhibitor of nitric oxide synthase; elevated levels indicate impaired endothelial function and are associated with increased stroke risk. Homocysteine (Hcy) contributes to stroke pathogenesis by promoting oxidative stress and vascular wall injury, and its levels are also commonly elevated in stroke patients. Among inflammatory markers, C-Reactive Protein (CRP) and High-Sensitivity C-Reactive Protein (hs-CRP) are sensitive indicators of systemic inflammation; elevated levels at admission predict larger infarct volume and poorer functional outcomes.
Specific markers of neuronal and glial cell injury include Neurofilament Light Chain (NFL) and Glial Fibrillary Acidic Protein (GFAP). NFL is an axon-specific intermediate filament protein rapidly released into the blood after brain tissue damage; it can be used to assess the degree of axonal injury after ischemic stroke and predict functional recovery. GFAP is a structural protein of astrocytes; elevated concentrations in serum or cerebrospinal fluid can help differentiate between ischemic and hemorrhagic stroke and correlate positively with hemorrhage volume and the degree of neurological deficit. S100 Calcium-Binding Protein B (S100B), primarily derived from astrocytes and melanocytes, shows significantly increased serum levels after acute brain injury and has been correlated with stroke severity and mortality. Neuron-Specific Enolase (NSE) is a marker enzyme for neurons and neuroendocrine cells; elevated serum NSE levels after a stroke reflect neuronal damage and are used for early diagnosis and prognosis assessment. Myelin Basic Protein (MBP) is a major structural component of central nervous system myelin; detection of MBP in the blood can indicate myelin destruction and axonal demyelination, holding potential value in assessing neuroremodeling and rehabilitation in the later stages of stroke.
While these biomarkers individually hold clinical value, a multi-marker panel strategy is more commonly employed in practice to enhance diagnostic sensitivity, specificity, and the ability to distinguish between stroke subtypes. From the awareness campaigns of World Stroke Day to interdisciplinary scientific innovation, the future promises real-time, dynamic monitoring of these biomarkers, providing a more precise biological basis for "early recognition, early intervention, and early rehabilitation." Stroke prevention and treatment are entering a new era characterized by "early identification, precision therapy, and comprehensive rehabilitation!"
References
1. Walter K. What is acute ischemic stroke? JAMA. 2022;327(9):885.
2. Xu A, Zhang H, Zhang Y, Wu J, Huang Z. Ischemic stroke and intervention strategies based on the timeline of stroke progression: Review and prospects. Acta Pharmaceutica Sinica B. Published online July 23, 2025.
3. Hilkens NA, Casolla B, Leung TW,et al. Stroke. Lancet,2024, 403(10446):2820-2836.
4. Aulin J, Sjlin K, Johan Lindb¦ck, et al. Neuroglial Biomarkers for Risk Assessment of Ischemic Stroke and Other Cardiovascular Events in Patients with Atrial Fibrillation Not Receiving Oral Anticoagulation. Journal of the American Heart Association. Published online November 11, 2024.
5. Hilkens NA, Casolla B, Leung TW, Frank-Erik de Leeuw. Stroke. Lancet. 2024;403(10446):2820-2836.
AntibodySystem provides Stroke-related products, delivering more tools and solutions for research.
Recombinant Protein
|
Catalog |
Product Name |
|
AHF99102 |
Recombinant Human CD87/PLAUR/uPAR Protein, C-His (Active) |
|
YHF99101 |
Recombinant Human CD87/PLAUR/uPAR Protein, N-His |
|
EHC94301 |
Recombinant Human BMP4 Protein, N-Fc |
|
YHD03101 |
Recombinant Human GFAP Protein, N-His |
|
YHD19202 |
Recombinant Human NT-proBNP Protein, N-His-Flag |
|
YHC38601 |
Recombinant Human ENO2/NSE Protein, N-His |
|
YHC08401 |
Recombinant Human S100B Protein, N-His |
|
YHB99401 |
Recombinant Human MBP Protein, N-His |
Antibody
|
Catalog |
Product Name |
|
FHF99110 |
Anti-Human CD87/PLAUR/uPAR Antibody (SAA2029) |
|
FHF99113 |
Anti-Human CD87/PLAUR/uPAR Antibody (SAA2029), APC |
|
DHF99101 |
Research Grade Anti-Human CD87/PLAUR/uPAR (ATN-658) |
|
PHC94301 |
Anti-BMP4 Polyclonal Antibody |
|
RHC94302 |
Anti-BMP4 Antibody (R3C93) |
|
RGD03101 |
Anti-Human GFAP Antibody (SAA0567) |
|
PHD03101 |
Anti-GFAP Polyclonal Antibody |
|
MHD19201 |
Anti-Human NT-ProBNP Monoclonal Antibody (1A344) |
|
MHJ48301 |
Anti-Human GDF15 Monoclonal Antibody (1A346) |
|
MHC38601 |
Anti-Human ENO2/NSE Monoclonal Antibody (1A303) |
|
RHC38605 |
Anti-ENO2/NSE Antibody (R3B53) |
|
MHC08408 |
Anti-Human S100B Monoclonal Antibody (1A295) |
|
RXX17102 |
Anti-MBP Antibody (R1B47) |
ELISA KIT
|
Catalog |
Product Name |
|
KHC08401 |
Human S100B ELISA Kit |
