The U.S. cruise ship "Serenade of the Seas," operated by Royal Caribbean, suddenly experienced an outbreak of Norovirus (NV). Nearly 100 individuals on board, including passengers and crew members, were infected. According to the U.S. Centers for Disease Control and Prevention (CDC), as of October 1, 94 out of 1,874 passengers exhibited symptoms of infection such as diarrhea and vomiting, while 4 out of 883 crew members also fell ill. However, the actual number of infections is likely higher, as many passengers reportedly chose to conceal their symptoms out of concern that reporting illness would lead to mandatory isolation in their cabins. The CDC stated that 19 similar incidents have been recorded so far in 2024, 14 of which were caused by Norovirus.

About Norovirus
Human Norovirus is a leading cause of gastroenteritis. It is estimated to cause approximately 677 million cases of acute gastroenteritis globally each year. Although children under five years of age are the primary target, individuals of all age groups are universally susceptible. Transmission routes include the fecal-oral route, indirect contact with environments contaminated by feces, as well as foodborne and waterborne transmission.
Typically, the high season for Norovirus spans from October to March of the following year. Cluster outbreaks occur predominantly in settings such as schools and childcare facilities. In crowded environments, Norovirus infection is characterized primarily by vomiting and diarrhea. While the illness is usually self-limiting with a short duration, it can lead to chronic diarrhea and even malnutrition in immunocompromised individuals. If a family member contracts Norovirus, disinfection is crucial for preventing intra-household transmission.
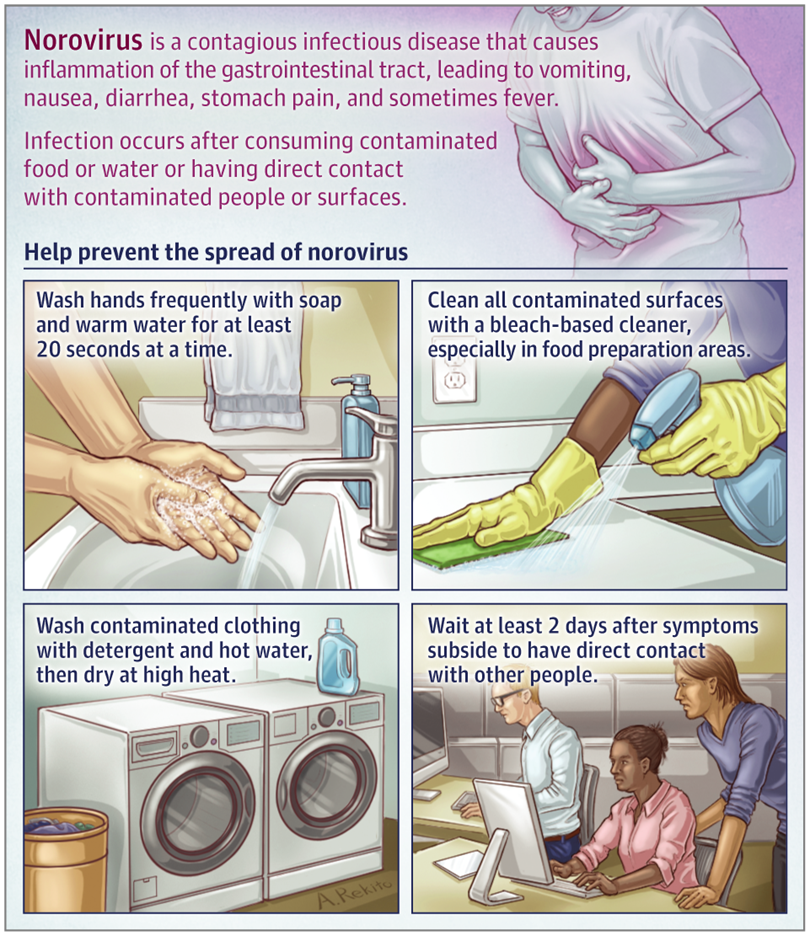
Figure 1. What Is Norovirus?(JAMA. 2019;322;(20):2032.)
Norovirus Structure
From a virological perspective, Norovirus belongs to the genus Norovirus within the family Caliciviridae. The viral particle is approximately 27-40 nm in diameter, with a genome length of about 7.5 kb. The Norovirus genome encodes six non-structural proteins (NSPs): p48 (NS1/2), p41 (NS3), p22 (NS4), VPg (NS5), protease (NS6), and RNA-dependent RNA polymerase (RdRP) (NS7), as well as the major structural protein VP1 and the minor structural protein VP2. The NSPs are primarily responsible for forming the viral replication complex, RNA synthesis, cleavage of the protein precursor, and host cell membrane and immune regulation. The structural protein VP1 assembles into a T=3 symmetrical capsid that forms the main framework of the viral particle, while VP2 assists in genome packaging inside the capsid and enhances particle stability.
Based on genetic characteristics, Noroviruses are classified into 10 genogroups and 49 genotypes. Five genogroups are associated with human infection (i.e., human Noroviruses): GI, GII, GIV, GVIII, and GIX. For over two decades, GII.4 has been the most common genotype causing Norovirus infection and disease outbreaks.
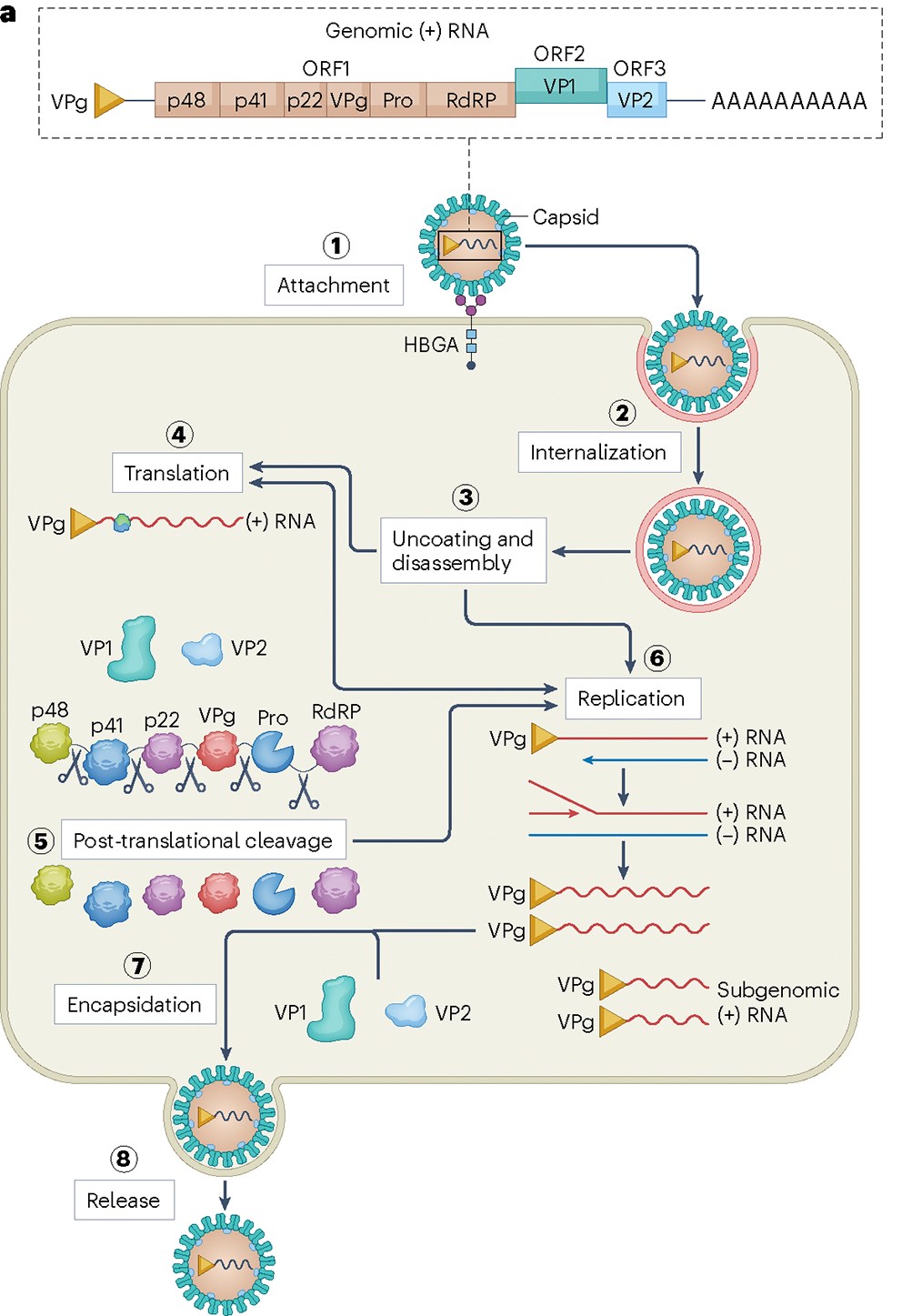
Figure 2. Human norovirus genome, entry, replication and structure(Nat Rev Microbiol. Author manuscript; available in PMC: 2025 Jun 1.)
Vaccine Research Progress
Confronting the persistent threat of Norovirus, vaccine development is considered crucial for prevention and control. However, to date, there are no approved vaccines or antiviral therapies specifically for Norovirus.
Although several candidate vaccines are currently in clinical trials globally, with more in preclinical stages, a major obstacle is the lack of a stable and reliable cell culture system, hindering the development of traditional inactivated or live attenuated vaccines. Consequently, Norovirus vaccine research is largely confined to recombinant technology routes. All vaccine candidates in clinical development target the major capsid protein VP1, employing three different protein expression strategies. Despite the challenging path ahead, in-depth research into the viral replication mechanisms and host interactions is fostering hope for new generation immunization strategies, represented by these candidate vaccines, for ultimately controlling Norovirus.
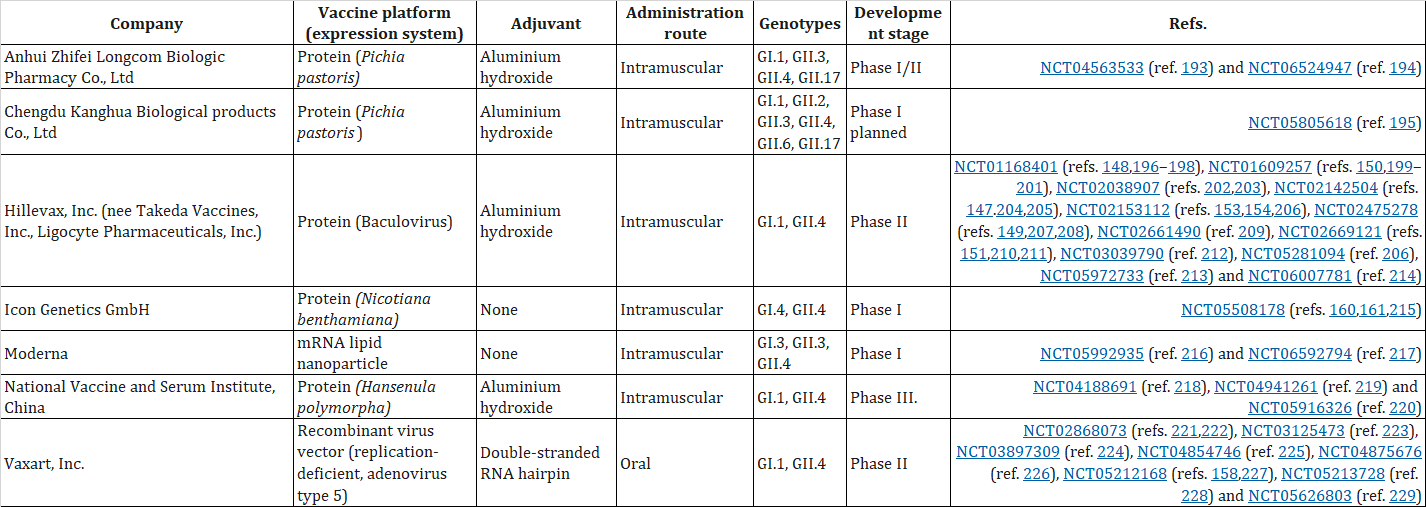
Figure 3. VP1-based norovirus vaccine candidates in clinical development(Nat Rev Microbiol. Author manuscript; available in PMC: 2025 Jun 1)
In summary, Norovirus, as a highly infectious and environmentally stable pathogen, poses an ongoing challenge to global public health through its frequent cluster outbreaks. Although no specific antiviral drugs or licensed vaccines are currently available, recombinant protein-based candidate vaccines show promising potential, driven by growing understanding of viral replication mechanisms, host interactions, and immune responses. Looking forward, key directions for effectively controlling Norovirus transmission and reducing its disease burden include accelerating the development of vaccines and related therapies through interdisciplinary collaboration, and strengthening public health surveillance and health education.
References
1. Desai AN. What Is Norovirus? JAMA. 2019;322(20):2032.
2. Cheng C, Cai X, Li J, Zhang X, Xie Y, Zhang J. In Vitro Culture of Human Norovirus in the Last 20 Years. Biomedicines. 2024;12(11):2442.
3. Prasad V, Atmar RL, Ramani S, et al. Norovirus replication, host interactions and vaccine advances. Nature Reviews Microbiology. Published online January 17, 2025.
4. Cates, J. E.; Vinjé, J.; Parashar, U.; Hall, A. J. Recent Advances in Human Norovirus Research and Implications for Candidate Vaccines. Expert Review of Vaccines 2020, 19 (6), 539–548.
AntibodySystem provides Norovirus products, delivering more tools and solutions for research.
|
Catalog |
Prouduct Name |
|
EVV25601 |
Recombinant Norovirus GII VP1/ORF2 Protein, C-His |
|
RVV25604 |
Anti-Norovirus/Norwalk virus Capsid protein/VP1 Nanobody (Nano-85) |
|
RVV25605 |
Anti-Norovirus/Norwalk virus Capsid protein VP1/p59 Antibody (5I2) |
|
RVV25606 |
Anti-Norovirus/Norwalk virus Capsid protein VP1/p59 Antibody (Nano-7) |
|
RVV25607 |
Anti-Norovirus/Norwalk virus Major capsid protein/VP1 Antibody (A1431) |
|
RVV33401 |
Anti-Mouse Norovirus Capsid protein Antibody (A6.2) |
|
RVV25601 |
Anti-Norovirus/Norwalk virus Capsid protein Antibody (5B18) |
|
RVV25602 |
Anti-Norovirus/Norwalk virus Capsid protein/VP1 Antibody (Nano-4) |
|
RVV25603 |
Anti-Norovirus/Norwalk virus Capsid protein/VP1 Antibody (Nano-25) |
|
RVV25608 |
Anti-Norovirus/Norwalk virus Major capsid protein/VP1 Antibody (A1227) |
